3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2005; 2(2):58-63. doi:10.7150/ijms.2.58 This issue Cite
Research Paper
A folate-rich diet is as effective as folic acid from supplements in decreasing plasma homocysteine concentrations
1. Unitat de Lípids i Arteriosclerosi, Servei de Medicina Interna, Hospital Universitari de Bellvitge, IDIBELL, c/Feixa Llarga s/n, 08907 Hospitalet de Llobregat, Barcelona, Spain.
2. Unitat de Metabolopaties. Servei de Bioquimica. Hospital de Sant Joan de Déu. Passeig Sant Joan de Déu, 2, 08950 Esplugues de Llobregat. Barcelona. Spain.
3. Departament de Genètica. Facultat de Biologia. Universitat de Barcelona. Avda. Diagonal 645, 08028 Barcelona. Spain.
4. Servei d'Angiologia i Cirurgia Vascular. Hospital Universitari de Bellvitge, IDIBELL, c/Feixa Llarga s/n, 08907 Hospitalet de Llobregat, Barcelona, Spain
Received 2004-9-1; Accepted 2005-2-1; Published 2005-4-1
Abstract
Background & Aims: At least 500 μg of folic acid are required daily to treat hyperhomocysteinemia. To reach this amount by dietary changes alone may be difficult because food has a low folic acid content and bioavailability. No studies have compared the effects of similar amounts of additional folate derived from a combination of folate-rich and fortified foods or folic acid from supplements on plasma total homocysteine (tHcy) concentrations, which was the aim of this study. Methods: Twenty male patients with hyperhomocysteinemia and coronary artery disease were included in a randomized, crossover intervention trial. Patients were treated daily with a combination of foods containing approximately 500 μg of folate or with one 500 μg capsule of synthetic folic acid over two five-week periods separated by a five-week wash-out period. Results: Plasma folate increased markedly (p<0.001) and plasma tHcy decreased (p<0.001) with both therapies. Folate-rich foods decreased tHcy by 8.6% (95% CI: –15.9 to –1.2) and synthetic folic acid capsules by 8% (95% CI: –13.3 to –2.7). Conclusions: This study shows, for the first time in the literature, that a folate-rich diet is as effective as folic acid capsules in decreasing plasma tHcy concentrations and adds further support to the recommendation of those diets to prevent cardiovascular disease.
Keywords: Folate, diet, folic acid, homocysteine.
1. INTRODUCTION
A graded and continuous relationship exists between plasma total homocysteine (tHcy) concentrations and the risk of cardiovascular disease [1,2] and the importance of tHcy measurement as screening test in the evaluation of cardiovascular risk is increasing [3]. Some evidence indicates that homocysteine-lowering therapy with folic acid may prevent cardiovascular disease [4,5] and improve arterial function [6,7]. Observational studies show that consumption of folate-rich foods, particularly fruit, vegetables and cereals, is inversely correlated with tHcy [8,9], and there is evidence that increasing fruit and vegetable consumption may induce a clinically significant decrease in tHcy [10]. However, as the content and bioavailability of folate in food are relatively low, it has been stated that diet is not an effective means of reducing tHcy concentrations [11,12] and daily doses of at least 400-500 μg of synthetic folic acid from supplements have been recommended to achieve a therapeutic effect [13, 14]. This study was designed to ascertain whether a moderate amount of folate derived from a combination of folate-rich and fortified foods is as effective as folic acid from supplements in decreasing plasma tHcy concentrations in patients with coronary artery disease (CAD) and hyperhomocysteinemia.
2. MATERIALS AND METHODS
Twenty-two male outpatients with a history of CAD (angina or myocardial infarction at least three months prior to inclusion) and hyperhomocysteinemia were initially included in the study. Hyperhomocysteinemia was arbitrarily defined as tHcy ≥ 12 μmol/l, considering that desirable values of tHcy in CAD patients have been defined as ≤ 10 μmol/l [11]. Two of the 22 patients were later excluded: one for a transient ischemic attack and the other for “feeling ill” during the treatment period with folate capsules. Characteristics of the subjects studied are shown in Table 1. No patient had gastrointestinal disorders or was under treatment with vitamin supplements or drugs that might affect folate metabolism. All subjects had previously been advised to follow a diet low in cholesterol and saturated fat, and none consumed foods fortified with folic acid. This study was approved by the Ethics Committee of the Hospital Universitari de Bellvitge and all participants gave written informed consent.
Design
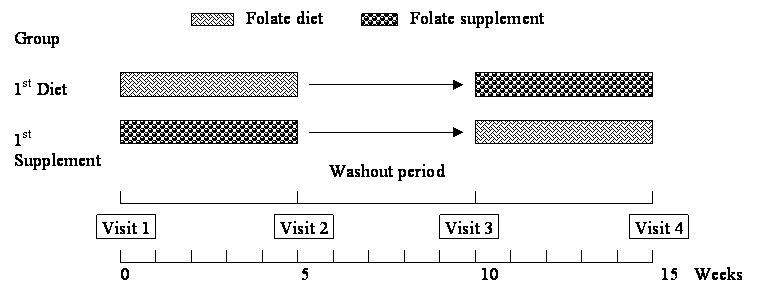
This study was designed as a randomized, crossover, dietary intervention trial. Two treatments were used: one consisted of a variety of foods which are good sources of folate and the other of capsules containing 500 μg of synthetic folic acid. The first treatment consisted of take-away food-packages containing approximately 500 μg of folate in the following items: 250 ml of orange juice; 140 g mixed greens and vegetables, including 90 g lettuce, 40 to 60 g of a mixture of 3 or more of the following vegetables: tomato, cucumber, carrots, red or green pepper, spinach, celery, watercress, avocado, radish, red cabbage or corn; 20 g nuts (hazelnuts, walnuts or almonds); 50 g beans (lentils, chickpeas, kidney beans or peas); fruits: 150 g kiwi or 200 g banana or tangerine, or 400 g apple or pear; and an average of 40 g regular Kellogg's Special K or All-Bran flakes. Seven different food combinations, one for each day of the week, were planned. Every daily food package contained around 370 μg of natural folate from food and 130 μg of synthetic folic acid from breakfast cereals. Participants were asked to maintain their usual eating pattern and were randomly allocated to consume one food package or to take one capsule daily for five weeks, followed by a five-week “washout” period during which they consumed only their usual diet. After this period, they were switched to the alternate treatment for another five weeks (Figure 1).
Adherence to the study treatment was carefully monitored. Unannounced 24-hour diet recalls were made at least three-times during the study. A self-administered three-day diet recall was also completed during the week prior to each visit. The nutrient content of the diets was estimated with the Food Processor Plus software, version 8.44 (ESHA Research, INC, 2000).
The main outcome of the study was the change in tHcy from baseline.
Laboratory determinations
Venous blood samples were collected after a 12-hour-fast. Aliquots were prepared and frozen at –40º C and analyzed within the next 2 weeks in the same laboratory (Hospital Sant Joan de Déu). Plasma tHcy was determined by HPLC, as previously described [15]. Within-run imprecision (n=18) was 3% (70.7±2.2 μmol/l) and 4% (8.9±0.3 μmol /l), and between-day imprecision (n=18) was 6% (6.9±0.4 μmol/l) and 6% (55.4±3.1 μmol/l). Vitamin B12 and folate levels were determined by radioassay (Simultrac, Becton Dickinson) and pyridoxal phosphate was measured by HPLC (Chromsystem kit).
The lipid profile included total cholesterol, triglyceride and apolipoproteins (Apo) A-1 and B in plasma, and cholesterol in high-density lipoproteins (HDLc). HDL were isolated by selective precipitation of lipoproteins with polyethylene glycol, and LDL cholesterol (LDLc) was calculated with the Friedewald formula. Triglyceride and cholesterol were measured using commercial colorimetric methods (Boehringer Manheim, Barcelona, Spain). ApoA‑1 and apoB concentrations were measured by radial immunodiffusion in agarose plaques. Fibrinogen was measured by a PT-derived turbidimetric method. For detection of the methylenetetrahydrofolate reductase (MTHFR) 677 C>T mutation, genomic DNA was prepared from peripheral blood leukocytes using a standard method. Primers for PCR amplification were those described by Frosst et al.[16]. The PCR product was digested with HinfI and analyzed by polyacrylamide gel electrophoresis.
Statistical analysis
Distribution of study variables was examined with the use of standard exploratory analytic techniques. Basal and follow-up values were measured at the beginning and at the end of each treatment phase, respectively. Multivariate analysis of variance (MANOVA) was used for comparison of the effects of treatment. Characteristics of the subjects first treated with dietary folate and those who first received folate supplements were compared with student's t test for independent samples and two-tailed Fisher's Exact Test; no significant differences were observed. The influence of treatment order on outcome variables was analyzed by MANOVA with three factors: sequence (first diet or first supplement) treatment (diet or supplement), and follow-up (basal or follow-up measures) and each dependent variable (tHcy, folic acid and cobalamin and lipid metabolism parameters) was analyzed separately. No relationship between treatment order and the effects of therapy was observed; therefore this variable was not considered in the statistical analysis. For all dependent variables, percent changes were calculated as the difference between baseline and follow-up measures divided by the baseline measure, with the result multiplied by 100. Pearson's coefficients were used to assess correlations between variables. Two-sided P values were used. SPSS software (version 10.0) was used for statistical analyses.
3. RESULTS
Baseline characteristics of the subjects are shown in Table 1. The homozygous 677 C>T mutation in the MTHFR gene was present in 20 % of patients. This proportion is higher than that observed in a former study of CAD patients from our institution [17], which may be explained by the fact that patients from this study were selected on the basis of high tHcy levels.
A statistically-significant tHcy-lowering effect (p<0.001) was seen with both folate-rich foods (-8.6%; 95% CI: –15.9 to –1.2) and folic acid from supplements (-8.0%; 95% CI: –13.3 to –2.7) (Table 2). No significant differences (p of interaction=0.883) were noted in the degree of tHcy lowering between the two forms of therapy. Both folate-rich foods and folic acid supplements increased folate concentration (p< 0.001), and this increase was more pronounced with folic acid supplements (p for interaction = 0.005). Serum cobalamin levels did not change significantly with either form of therapy. Baseline tHcy values were significantly and inversely related to tHcy changes (r= - 0.89, p< 0.001, and r= -0.57, p= 0.014 during diet and supplement periods, respectively).
Both folate-rich foods and folic acid supplements decreased plasma LDLc (-12.2%; -21.8 - -2.6% and -15.0%; -23.5 - -6.4%, respectively), HDLc (-5.9%; -10.4 - -1.4% and -2.1%; -6.9 - 2.7%, respectively), apoA-1 (-3.8%; -11.3% - 3.8% and -0.9%; -8.1% - 6.3%, respectively) and apoB (-6.6%; -13.0 - -0.1% and -4.8%; -9.4% - 0.3%, respectively). No significant changes were noted in serum triglyceride, glucose, and plasma fibrinogen concentrations. A small, but not clinically-significant decrease in body mass index was seen with both therapies.
Daily energy intake was similar with the two interventions. Protein intake decreased during the period of diet therapy (-8.3%), and slightly increased (1.8%) with supplement therapy; the difference between the changes induced by both therapies was significant (p= 0.025). Carbohydrate intake increased with diet therapy (10.8%), and slightly decreased (-2.5%) during supplement therapy (p=0.017). No significant differences between the two treatments were found in the amount of total fat and cholesterol intake, whereas fiber intake increased more markedly during diet therapy than during supplement therapy (39.3% vs. 2.4%; p=0.018). The absolute dietary intake of B vitamins during both treatment periods is shown in Table 3. The intake of folate from food increased significantly with diet therapy, but no changes were seen during supplement therapy. Vitamin B6 intake increased (p< 0.001) with both interventions, but the increase was greater during diet therapy than during synthetic folate treatment (p for interaction=0.017), whereas vitamin B12 intake did not change with either form of therapy.
4. DISCUSSION
This study shows for the first time that a moderate daily intake of a variety of folate-rich foods is as effective as folic acid from supplements for lowering plasma tHcy concentrations. A high percentage of patients with CAD has hyperhomocysteinemia [17] and low folate intake with ensuing low folate serum concentrations is the main cause of this disorder [18].
A dietary folate intake of 350 μg/day is recommended in the general adult population, but most persons in developed countries do not attain this daily amount from diet alone. In Europe, mean dietary folate intake in adults is 291 μg (range:197-326) for men and 247 μg (range 168-320) for women [19]. A reasonable approach for increasing folate intake is to include larger amounts of folate-rich foods in the diet, i.e. leafy green vegetables, fruits, legumes and ready-to-eat fortified cereals. Such an approach was used in this study by supplying participants with a moderate amount of folate-rich foods providing an average of 500 μg folate daily. This dose of folate was selected since it was higher than the minimal effective dose of 0.2 mg/day found to decrease homocysteine (20), and higher than the mean dietary folate intake in European men, but not high enough to be attained with a folate-rich diet.
The toxicity of folic acid is extremely low even after prolonged use of high doses (21). It has been shown that folic acid in doses between 0.2 and 15 mg/day can lower plasma homocysteine levels without apparent toxicity. A main safety issue is the risk of neurologic disorders when high doses of folic acid are used in the presence of an underlying vitamin B12 deficiency. However, it is customary to rule out vitamin B12 deficiency before giving supplemental folate. Once folate therapy is initiated, it is usually maintained on a long-term basis, but until results of controlled clinical trials become available, no firm recommendations regarding that therapy can be made.
Owing to the relative differences in bioavailability of naturally-occurring food folate and the more bioavailable synthetic folic acid, 100 μg of natural folate from unfortified foods has been equated to 60 μg of synthetic folic acid from fortified food and to 50 μg of synthetic folic acid from supplements [22]. However, studies comparing the effects of folate from food with those of supplements on tHcy are scant. Brouwer et al [23] reported that a diet including 560 μg folate per day decreased tHcy by a mean of 2 μmol/l (95% CI: 1.0-3.0), and a similar effect was observed with 500 μg/day folic acid from supplements. Ridell et al [24] reported that folate from both supplements and breakfast cereals had a greater effect on tHcy than folate-rich foods. In a meta-analysis of randomized trials that assessed the tHcy-lowering effect of folic acid-based supplements, a clear heterogeneity among trials was also seen and was not explained by differences in age, sex or duration of treatment, but by differences in MTHFR gene polymorphism or pre-treatment tHcy and folate concentrations [13, 25]. Nutritional interactions that affect folate bioavailability may also have influenced the different tHcy-lowering effects of diet therapy among studies.
The design of this study – an outpatient crossover feeding trial – entailed some difficulties in ensuring compliance. However, these difficulties were partially offset by detailed dietary instructions and reinforcement at each visit during the trial. The amount of fruit, vegetables, beans, nuts and breakfast cereals given to participants fits with what could be considered a reasonable usual daily intake. This contrasts with the higher amounts of folate-rich foods that were used in other studies [23]. We were able to use smaller amounts of fruit and vegetables to supply the 500 μg folate per day because other foods, such as beans, nuts and folic acid-enriched breakfast cereals were also included as a source of folate. This type of diet is easier to follow on a long-term basis, and may be useful in a general population approach to increasing the intake of folate.
We observed a decrease in LDL-C during both the folate-rich diet and the folic acid supplement periods. The increase in intake of fruit, vegetables, beans, nuts and breakfast cereals during the folate-rich diet could explain these lipid changes since these foods have a cholesterol-lowering effect, both for LDL and HDL fractions, owing to their high carbohydrate and fiber content. However, the decrease in LDL-C during the folic acid supplement period cannot be attributed to the small dietary changes observed. Possibly, the closer physician and dietitian supervision promoted a healthier overall life-style resulting beneficial lipid effects.
A meta-analysis of observational studies of blood tHcy and vascular disease indicated that prolonged lowering of tHcy concentration by 1 μmol/l is associated with approximately a 10% reduction in risk throughout the range of 10-15 μmol/l [26], and that the benefit of decreasing tHcy is greater in patients with CAD than in the general population [27]. In this study, folate therapy from either diet or supplements decreased mean tHcy by more than 1 μmol/l, and this effect may be clearly beneficial for preventing recurrences and death in patients with CAD.
5. CONCLUSIONS
To our knowledge, this is the first study in the international literature to compare the effects of similar amounts of additional folate derived from a combination of folate-rich foods and fortified breakfast cereals, or folic acid from supplements, on tHcy concentrations. The results suggest that diet is as effective and feasible as supplementation for lowering tHcy, and further support the recommendation of diets with a variety of folate-rich foods for lowering tHcy and preventing cardiovascular disease.
Acknowledgements
This study was supported by a grant from the Foundation La Marató de TV3, Barcelona, Spain and from the Foundation for the Investigation and Prevention of Cardiovascular Diseases (FIPEC), Barcelona, Spain.
The authors thank E. Ros for conceptual review of the manuscript; R. Urreitzi for skillful technical assistance; N. Virgili, A. Pérez-Heras and M. Serra for the analysis of diet records; and Christine O'Hara for correction of the English version. We also thank Kellogg's España for supplying the breakfast cereals and financial support in the analysis of diet records.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Eikelboom JW, Lonn E, Genest J Jr, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med. 1999 ;131(5):363-75
2. Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Br Med J. 2002 ;325(7574):1202-6
3. Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J. et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin Chem. 2004 ;50(1):3-32
4. Vermeulen EG, Stehouwer CD, Twisk JW, van den Berg M, de Jong SC, Mackaay AJ, van Campen CM, Visser FC, Jakobs CA, Bulterjis EJ, Rauwerda JA. Effect of homocysteine-lowering treatment with folic acid plus vitamin B6 on progression of subclinical atherosclerosis: a randomized, placebo-controlled trial. Lancet. 2000 ;355(9203):517-22
5. Potena L, Grigioni F, Magnani G, Sorbello S, Sassi S, Poci MG. et al. Folate supplementation after heart transplantation: effects on homocysteine plasma levels and allograft vascular disease. Clin Nutr. 2002 ;21(3):245-8
6. Holven KB, Holm T, Aukrust P, Christensen B, Kjekshus J, Andreassen AK, Gullestad L, Hagve TA, Svilaas A, Ose L, Nenseter MS. Effect of folic acid on endothelium-dependent vasodilation and nitric oxid-derived products in hyperhomocysteinemiac subjects. Am J Med. 2001 ;110(7):536-42
7. Woo KS, Chook P, Chan LL, Cheung AS, Fung WH, Qiao M, Lolin YI, Thomas GN, Sanderson JE, Metreweli C, Celermajer DS. Long-term improvement in homocysteine levels and arterial endothelial function after 1-year folic acid supplementation. Am J Med. 2002 ;112(7):535-9
8. Tucker KL, Selhub J, Wilson PW, Rosenberg IH. Dietary intake pattern relates to plasma folate and homocysteine concentrations. J Nutr. 1996 ;126(12):3025-31
9. Shimakawa T, Nieto FJ, Malinow MR, Chambless LE, Schreiner PJ, Szklo M. Vitamin intake: a possible determinant of plasma homocyst(e)ine among middle-aged adults. Ann Epidemiol. 1997 ;7(4):285-93
10. Broekmans WM, Klopping-Ketelaars IA, Schuurman CR, Verhagen H, van den Berg H, Kok FJ, van Poppel G. Fruits and vegetables increase plasma carotenoids and vitamins and decrease homocysteine in humans. J Nutr. 2000 ;130(6):1578-83
11. Omenn GS, Beresford SAA, Motulsky AG. Preventing coronary heart disease. B vitamins and homocysteine. Circulation. 1998 ;97(5):421-424
12. Ubbink JB, van der Merwe A, Vermaak WJ, Delport R. Hyperhomocysteinemia and the response to vitamin supplementation. Clin Investig. 1993 ;71(12):993-8
13. Homocysteine Lowering Trialists' Collaboration. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomized trials. Br Med J. 1998 ;316(7135):894-8
14. Mohan IV, Stansby G. Nutritional hyperhomocysteinemia. Br Med J. 1999 ;318(7198):1569-70
15. Vilaseca MA, Moyano D, Ferrer I, Artuch R. Total homocysteine in pediatric patients. Clin Chem. 1997 ;43(4):690-2
16. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP. et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995 ;10(1):111-3
17. Pinto X, Vilaseca MA, Garcia-Giralt N, Ferrer I, Pala M, Meco JF, Mainou C, Ordovas JM, Grinberg D, Balcells S; Baix Llobregat Homocysteine Study Group. Homocysteine and the MTHFR 677C→T allele in premature coronary artery disease. Case control and family studies. Eur J Clin Invest. 2001 ;31(1):24-30
18. Selhub J, Jacques PF, Rosenberg IH, Rogers G, Bowman BA, Gunter EW, Wright JD, Johnson CL. Serum total homocysteine concentrations in the Third National Health and Nutrition Examination Survey (1991-1994): Population reference ranges and contribution of vitamin status to high serum concentrations. Ann Intern Med. 1999 ;131(5):331-9
19. de Bree A, van Dusseldorp M, Brouwer IA, van het Hof KH, Steegers-Theunissen RP. Folate intake in Europe: recommended, actual and desired intake. Eur J Clin Nutr. 1997 ;51(10):643-60
20. Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: A statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1999 ;99(1):178-82
21. Butterworth CE, Tamura T. Folic acid safety and toxicity: a brief review. Am J Clin Nutr. 1989 ;50(2):353-8
22. Suitor CW, Bailey LB. Dietary folate equivalents: Interpretation and application. J Am Diet Assoc. 2000 ;100(1):88-94
23. Brouwer IA, van Dusseldorp M, Thomas CM, Duran M, Hautvast JG, Eskes TK, Steegers-Theunissen RP. Low-dose folic acid supplementation decreases plasma homocysteine concentrations: a randomized trial. Am J clin Nutr. 1999 ;69(1):99-104
24. Riddell LJ, Chisholm A, Williams S, Mann JI. Dietary strategies for lowering homocysteine concentrations. Am J Clin Nutr. 2000 ;71(6):1448-54
25. Malinow MR, Nieto FJ, Kruger WD, Duell PB, Hess DL, Gluckman RA, Block PC, Holzgang CR, Anderson PH, Seltzer D, Upson B, Lin QR. The effects of folic acid supplementation on plasma total homocysteine are modulated by multivitamin use and methylenetetrahydrofolate reductase genotypes. Arterioscler Thromb Vasc Biol. 1997 ;17(6):1157-62
26. Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995 ;274(13):1049-57
27. Vollset SE, Refsum H, Tverdal A, Nygard O, Nordrehaug JE, Tell GS, Ueland PM. Plasma total homocysteine and noncardiovascular mortality: the Hordaland Homocysteine Study. Am J Clin Nutr. 2001 ;74(1):130-6
Figures and Tables
Diagram of experimental design
Baseline characteristics of the study patients.
| Variable | All subjects(n=20) |
| Age (years) | 60.9 ± 8.91 |
| Body mass index (Kg/m2) | 28.6 ± 3.24 |
| History of hypertension n.(%) | 9 (45%) |
| History of diabetes n.(%) | 1 (5%) |
| Cardiovascular history Angor pectoris n. (%) Myocardial infarction n. (%) | 15 (75%)10 (50%) |
| Current smoker n.(%) | 2 (10%) |
| Total cholesterol (mmol/l) | 4.8 ± 0.69 |
| Triglyceride (mmol/l) | 1.3 ± 0.44 |
| LDL cholesterol (mmol/l) | 2.8 ± 0.66 |
| HDL cholesterol (mmol/l) | 1.3 ± 0.36 |
| Folate (nmol/l) | 16.6 ± 6.63 |
| Cobalamin (pmol/l) | 393.6 ± 168.7 |
| Homocysteine (μmol/l) | 16.1 ± 7.40 |
| MTHFR TT mutation | 4 (20%) |
± values are: means ± SD
Effect of dietary folate and folic acid from supplements on plasma total homocysteine, folate and cobalamin.
| Variable | Baseline | On-treatment | Treatment effect, mean percent change (95% CI) | p* | p** | p*** | |
| Homocysteine (μmol/l) | Folate-rich diet | 15.56 ± 3.05 | 14.18 ± 3.41 | -8.6%(-15.9;-1.2) | <0.001 | 0.008 | 0.883 |
| Folic acid from supplements | 14.32 ± 3.37 | 13.06 ± 2.77 | -8.0%(-13.3;-2.7) | ||||
| Folate (nmol/l) | Folate-rich diet | 18.89 ± 6.46 | 27.55 ± 9.48 | 52%(28.7;75.4) | <0.001 | 0.038 | 0.005 |
| Folic acid from supplements | 19.42 ± 7.53 | 34.53 ± 10.12 | 87%(68.7;106) | ||||
| Cobalamin (pmol/l) | Folate-rich diet | 401.6 ± 152.6 | 402.0 ± 180.6 | -0.94%(-7.5;5.6) | 0.350 | 0.700 | 0.358 |
| Folic acid from supplements | 405.7 ± 185.0 | 388.1 ± 140.1 | 0.15%(-5.8;6.2) |
CI: Confidence interval
* P value of ANOVA for follow up (baseline/on-treatment) comparison.
** P value of ANOVA for treatment (diet/supplements) comparison.
*** P value of ANOVA for interaction (follow up*treatment).
± values are: means ± SD
Dietary intake of B vitamins during the period of diet and supplement therapy.
| Variable | Baseline | On-treatment | Treatment effect, mean percent change (95% CI) | p* | p** | p*** | |
| Folate (μg/day) | Folate-rich diet | 335 ± 70 | 528 ± 63 | 66.5%(37.2;95.9) | 0.002 | <0.001 | 0.002 |
| Folic acid from supplements | 339 ± 106 | 316 ± 123 | 3.8%(-29.5;37.1) | ||||
| Vitamin B6 (mg/day) | Folate-rich diet | 1.89 ± 0.37 | 2.67 ± 0.5 | 45.5%(23.6;67.4) | <0.001 | 0.142 | 0.017 |
| Folic acid from supplements | 1.95 ± 0.55 | 2.23 ± 0.71 | 16.0%(1.6;30.4) | ||||
| Vitamin B12 (μg/day) | Folate-rich diet | 7.94 ± 6.49 | 7.04 ± 5.62 | 42.4%(-52.8;137.6) | 0.683 | 0.330 | 0.156 |
| Folic acid from supplements | 7.17 ± 4.49 | 9.79 ± 7.17 | 62.7%(-12.3;137.8) |
CI: Confidence interval
* P value of ANOVA for follow up (baseline/on-treatment) comparison.
** P value of ANOVA for treatment (diet/supplements) comparison.
*** P value of ANOVA for interaction (follow up*treatment).
± values are: means ± SD
Author biography
Xavier Pintó was born in Barcelona in 1956. He graduated in Medicine in 1980 at University of Barcelona and became Internal Medicine specialist after a 5-year residency at Hospital Universitario de Bellvitge. He completed a Doctoral thesis on lipoprotein and apolipoprotein abnormalities in patients with cerebral, coronary and peripheral atherosclerosis in 1988 at University of Barcelona. He is the Director of the Lipid and Atherosclerosis Unit of the Internal Medicine Service at the Hospital Universitario de Bellvitge and Associate Professor of Medicine at the University of Barcelona. Dr. Pintó research focuses on hiperlipidèmia, atherosclerosis, lipid-lowering agents and non-traditional cardiovascular risk factors, with special interest in homocysteine. He is a founding member of the Spanish Atherosclerosis Society and is on the editorial boards of the journals Clinica e Investigación en Arteriosclerosis and Medicina Clínica (Barc.). He is also a regular participant in different national committees. His research has resulted in the publication of more than 50 scientific papers, 11 book chapters and two books.
![]() Corresponding address:
Corresponding address:
Xavier Pintó, Unitat de Lípids i Arteriosclerosi, Servei de Medicina Interna, Hospital Universitario de Bellvitge, IDIBELL 08907 Hospitalet de Llobregat, Barcelona, Spain. Tel. 00 34 932607664. Fax 00 34 932607881. E-mail: xpintoscs.es

 Global reach, higher impact
Global reach, higher impact