3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2005; 2(1):30-35. doi:10.7150/ijms.2.30 This issue Cite
Research Paper
High level expression of apoptosis inhibitor in hepatoma cell line expressing Hepatitis B virus
Drexel Institute for Biotechnology and Virology Research, Department of Microbiology and Immunology, School of Medicine, Drexel University, Doylestown, PA 18901, USA
Received 2004-11-1; Accepted 2005-1-1; Published 2005-1-5
Abstract
The serious result of hepatitis B (HBV) virus infection is development of hepatocellular carcinoma (HCC). However, the reason of development of HCC in HBV infected patients is still unclear. Recently, the suppression of cell apoptosis is found to relate with the development of cell carcinogenesis, therefore, the expression of apoptosis inhibitor in the virus related cancer line such as hepatoma cell line HepG2.215 was investigated. There are at least six Human apoptosis inhibitors (IAP) have been identified now. They are cIAP1, cIAP2, XIAP, NAPI, survivin and pIAP. Using gene-assay technology, we have recently compared the expression of IAPs in the HepG2.215 cells that persistently expresses Hepatitis B virus by integrated HBV genome with its parent cell line HepG2. The results suggest that there was obviously increase of cIAP2 and cIAP1 in the HepG2.215 cells versus HepG2 cells. Those observations imply a possibility of long time HBV infection could induce the over-expressing apoptosis inhibitors, furthermore, causing the liver cancer. The high expression of cIAP1 and cIAP2 in HBV expressing cells was confirmed by RT-PCR and Northern blot analysis. However, we did not find the change of NIAP and suvivin in HepG2.215 cells. In contrast, the expression of XIAP was down in the HepG2.215 cells comparing with HepG2 cells. How HBV triggers the over-expression of apoptosis inhibitor is unclear. Transient transfection of HepG2 cells with the plasmids expressing different HBV proteins such as S, M, L, X and core proteins did not give a decisive conclusion. Further study is going on now.
Keywords: Apoptosis inhibitor, Apoptosis, cancer, Hepatitis B virus, Hepatoma cell
1. Introduction
Hepatitis B virus (HBV) is a major human pathogen responsible for acute and chronic liver disease. Worldwide, more than 350 million people are chronically infected with HBV and more than one third of these individuals will develop into serious liver diseases such as primary hepatocellular carcinoma (HCC), if left untreated, which causes an estimated 1 million deaths annually [4]. Therefore, clarification of the relationship of HBV infection and the development of HCC, consequently developing the therapeutic method, is imperative.
HBV is a small circular DNA virus, containing a nucleocapsid and an envelope. HBV nucleocapsid contains a relatively small incomplete double stranded DNA genome, viral polymerase and core protein. Its envelope is composed of viral surface proteins enclosed by a lipid membrane derived from host cells [11, 38], named LHBs, MHBs and SHBs, respectively to Large, middle and small surface protein [22]. Besides those viral proteins, HBV expresses a small non-structure protean named X protein, its function is still unclear. The role of HBV protein, particular of middle surface protein and X protein, in cell carcinogenesis is suggested [6, 13, 14, 37, 17, 27], but is far away to the conclusion. Therefore, further investigation is needed.
Recently, the studies have shown that the apoptotic cell death plays an important physiological role for normal cell development and tissue homeostasis. Dysregulation of apoptosis has been implicated in carcinogenesis, tumor progression and resistance of tumor cells to radio-and chemotherapy [28]. The molecular pathways leading to apoptosis are evolutionarily conserved and controlled by proteins that either promote or inhibit activation of a cascade of intracellular cysteine proteases known as caspases. Caspases can be divided into two groups based on the length of their prodomain and substrate specificity. The initiator caspase group includes caspase-2, caspase-8, caspase-9, and caspase-10, having long NH2-terminal prodomains, which interact with adapter molecules to form a death-inducing signaling complex. Downstream caspases such as caspase-3, caspase-6, and caspase-7 are executioner caspases that remain dormant until the initiator caspases activate them by proteolysis [8]. The activated executioner caspases cleave a number of structural and regulatory proteins, leading to apoptotic cell death [26].
One kind of proteins that regulate cell apoptosis are named Apoptosis inhibitors (IAP), which include c-IAP1, c-IAP2, XIAP, NAIP, survivin, and currently discovered pIAP [8, 9, 15, 16, 20, 21, 25, 30, 31]. These proteins contain a novel 80 amino acid motif that is defined as the Baculovirus IAP repeat (BIR) [20], which probably prevents the proteolytic processing of procaspase-3, procaspase-6, and procaspase-7 by binding and blocking the activity of caspase-9. Increasing evidence demonstrated that IAPs are up regulated in many human tumor types and tumor cell lines [ 18, 32, 29, 39, 40] such as pancreatic carcinoma cells, lung cancer cells [7, 10 ], prostate cancer cells [24] and renal carcinoma cell line [28, 19, 44 ]. Other interesting finding is that the cancer cells resistant to the radio- and chemotherapy have obviously high level of IAP [3, 19, 36]. However, the reason why these IAPs are over-expressed in tumor cell is unknown.
Since HBV is a tumor trigger, we are interested in whether HBV could stimulate the over-expression of IAPs. Therefore, we have compared the IAPs expression in the persistently HBV expressing cell line HepG2.215 and its parent cell line, a non-HBV expressing cell line HepG2. We found that cIAP1 and cIAP2 were clearly increased in the HBV expressing cells but XIAP was down regulated. The other two IAPs, NIAP and suvivin, remain unchanged. The results suggest that the long time stimulation of HBV viral proteins indeed changes the expression of apoptosis profile of host cells, which probably results in the carcinogenesis of liver cells.
2. Materials and methods
Cell lines and isolation of RNA
HepG2 cell line was bought from ATCC and HepG2.215 cell line, which persistently produces Hepatitis B virus due to the integrated HBV genome, was kindly provided by Dr. Acs (Mt. Sinai Medical College, NY, NY). Both cell lines have been maintained in our laboratory for more than 8 years.
Total RNA was isolated from 107 HepG2 cells or HepG2.215 cells using total RNA isolation Kit (Stratagene La Jolla, CA USA) as manufactory instruction. Briefly, cells were lysed with lysis buffer provided with Kit. Cell debris was removed by filtration. RNA was precipitated with ethanol and caught by RNA binding spin cup. After washing, DNA was degraded by the digestion of DNase. Finally RNA was released from cup and stored in –70° C for use.
Micro-array analysis
For gene array analysis, 100 μg RNA was reversely transcripted in to cDNA. cDNA fragments from mRNAs were fluorescently labeled and hybridized with chips contained more than 1,200 relative genes. Signal was detected and quantified. Mergen LTD (San Francisco, CA) performed the gene array analysis and result comparisons.
Reverse-transcription PCR detection of IAPs
One microgram RNA was used for the reverse-transcription PCR (RT-PCR). The reverse transcription was performed using RT-PCR kit (Stratagene, La Jolla, CA, USA), following by PCR amplification as described in our paper [22]. The primers with the sequence 3'-GAGGAGACAGTCCTACTGAAA (API1) and 3'-CATAGCATTATCCTTCGGTTC (API2) were used to detect cIAP2. Primers with the sequence 3'-GGGAAGCAGAGATCATTTTGC (API3) and 3'- AACTGAGTATATCCATGTCCC (API4) were used to detect XIAP. PCR bands were resolved in 1% agarose gel and quantified by software of AlphaIntertech.
Northern Blot detection of IAPs RNA
Ten micro grams RNA was resolved by 1% agarose gel electrophoresis. RNA was transferred to nylon membrane for Northern blot analysis. “Northern” type blotting was performed essentially as described by Lu et al. [23]. The probe was made from PCR amplification of the plasmid containing IAPs sequence. Briefly, the plasmid containing IAPs DNA sequence was used as a template for PCR amplification. PCR was performed as described before except for 20μCi 32-P dCTP was used in place of the non-radioactive dCTP [23]. 32-P labeled IAP fragments were purified by Probe Quant G50 micro column (Amersham, Piscataway, NJ). The membranes were hybridized with IAP probe (>107 cpm/ml) at 68° C, overnight. The images were acquired by phosphorimager.
Detection of IAP proteins by immune-precipitation with anti-IAP antibody
HepG2.2.15 cells or HepG2 cells were labeled by 35-S methionine (Amersham, Piscataway. NJ) as described in Lu et al. [23]. Briefly, 107 cells were washed with phosphate-buffered saline (PBS) three times, and incubated with 3 ml methionine minus RPMI 1640 medium 30 minutes. Cells were labeled with 100μCi/ml 35-S methionine overnight. After washing with PBS, cells were released by trypsin digestion and then were lysed with 0.8ml Tris-HCl 0.05M pH 7.5, NaCl 0.15 M, MgCl2 0.005M, Np-40 0.2% at room temperature for 30 minutes. The nuclei and cell debris were removed by centrifugation at 14,000 rpm 5 minutes. The lysate was collected and the radioactivity was determined by Trichloroacetic acid (TCA) precipitation. For protein analysis, 100μl cell lysate was immune-precipitated with 30μl protein G beads (Roche, Switzerland) at 4° C overnight, which pre-absorbed with monoclonal anti-IAP antibody (1μg/ ml) (Zymed, Poli Alto, CA). After 4 washings with PBS, 0.05% Tween 20, the IAP proteins were released from beads through cooking at 95° C, 10 minutes in 20 μl sample buffer and resolved by 12.5 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to polyvinyl difluoride (PVDF) membrane (Bio-Rad, Hercules CA) and images were analyzed by Phosphorimager.
3. Results
The profile of IAPs expression in the cells producing HBV
The change of IAPs expression in the HBV expressing cells was first investigated by the comparison of the gene expression profile in the HepG2.215 and HepG2 cells using gene array technology.
HepG2.215 is a cell line that derives from HepG2. The only difference between them is HepG2.215 cell is able to produce infectious viruses through the HBV genome integrated in the cell chromosome [1, 34, 35]. The results from gene array suggest that the expression of cIAP1 and cIAP2 genes was obviously higher in the HepG2.215 cells than in the HepG2 cells, approximately 1.6 folds and 9 folds respectively (Table 1). The strong intensity of the dots implies that the increase of the expression of cIAP2 and cIAP1 most likely are reliable. In contrast, the gene expression of XIAP seemed to be 21 folds down in the HepG2.215 cells versus in the HepG2 cells (Table 1). However, the low expression of XIAP in the samples (negative intensity of the dot) probably lessens the significant of this difference. We will discuss this in detail late. The expression of two other IAPs: NAIP and survivin, have not shown any changes by gene array (Table 1).
Analysis of IAPs expression in the HepG2.215 and HepG2 cells
| HepG215/HepG2 (Fold) | Intensity of dots | |
| cIAP2 | 9.00 | 9.0357/-6.739 |
| cIAP1 | 1.60 | 67.037/35.824 |
| XIAP | -21.9 | -5.354/0.0414 |
| NIAP | 1.00 | -8.353/-11.45 |
| Survivin | 1.00 | -11.844/-8.3526 |
One hundred micrograms RNA from HepG2.215 and HepG2 cells were analyzed by gene array (Mergen LTD). The intensity of dots were calculated and standardized by computer. The comparison was then performed.
Because the expressions of cIAP2 and XIAP in the cell line expressing HBV were dramatically altered, therefore, our next investigation focused in these two species.
The up-regulation of cIAP2 in cells expressing HBV
The over-expression of cIAP2 in the HBV expressing cell line, HepG2.215. The RNA from HepG2.215 and HepG2 cells were isolated. The expression of cIAP2 was examined with RT-PCR and Northern blot. A. RT-PCR detects cIAP2. One microgram RNA from HepG2 (number 1, duplicate) and HepG2.215 cells (number 2, duplicate) were reverse- transcripted, and then amplified by PCR. The result from PCR was shown as a 434bp band. β-Actin was used as a loading control. B. Northern blot detects cIAP2. Ten microgram RNA from HepG2 (number 1, duplicate) and HepG2.215 cells (number 2, duplicate) were separated by denature gel, then hybridized with cIAP2 specific probe. The cIAP2 and cIAP1 bands were indicated. β-Actin was used as a loading control as well.
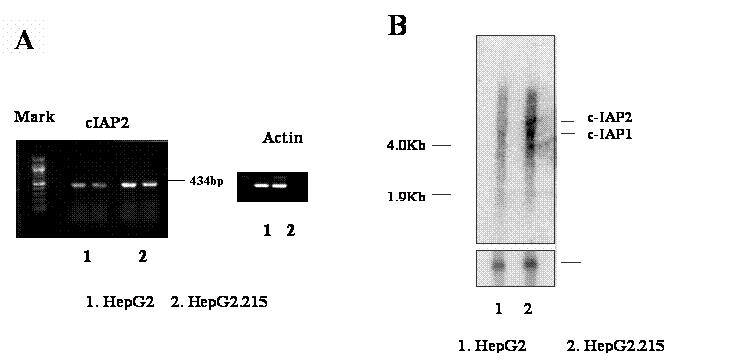
The increase of gene expression of cIAP2 in HepG2.215 cells was further investigated by the reverse transcription PCR (RT-PCR) and Northern Blot. Using the specific primer set of IAP1/ IAP2 (See Materials and Methods), which amplifies the cAPI2 gene fragment from coding number 681 to 1014, a corresponding band with 434 base pairs was generated (Figure 1A). Figure 1A shows that the expression of cIAP2 was obviously higher in the HepG2.215, a cell line expressing HBV, than in the HepG2, a cell line absence of HBV (Figure 1A, 1&2). The up-regulation of cIAP2 expression in HepG2.215 cells is not due to an impact upon loading, at the same condition, the expression of β-Actin in both cell lines remained unchanged (Figure 1A). The up-regulation of cIAP2 expression is consistent with the result from gene array analysis.
The up-regulation of cIAP2 was confirmed by the Northern Blot analysis. After the hybridization with the probe specifying cIAP2, cIAP2 RNA appeared as a special band located in the place with the molecular weight around 8 kilo-base pair (Kb) (Figure 1B). Because the cIAP2 and cIAP1 have 80% homogenous in their DNA sequence, the probe used to detect cIAP2 may detect the cIAP1 either [31, 43]. Therefore, the band with molecular weight around 4.5 kilo base pairs detected by cIAP2 probe was expected as the cIAP1 RNA (Figure 1B, the band below cIAP2 band). The nature of the third band below cIAP1 is unknown. However, it was also found in the testis cancer cells by Holcik et al. [15]. This band is studying in our laboratory now.
Comparing with the HepG2 cells, the RNA level of cIAP2 in the HepG2.215 cells was obviously higher by the Northern Blot (Figure 1B, lane 2). This is agreement with the previous results from RT-PCR and gene array analysis. However, the increase of expression of cIAP1 was also can be observed in the HepG2.215 cells by Northern Blot (Figure 1B, lane 2), despite the cIAP1 expressing level was not stronger as cIAP2. The increase of the expression of cIAP2 and cIAP1 in the HepG2.215 cells were not due to the unequal loading of the samples, this is supported by the fact that the expression of the β-actin RNA in these samples was unchanged (Figure 1B).
Detection of XIAP with RT-PCT and Northern Blot
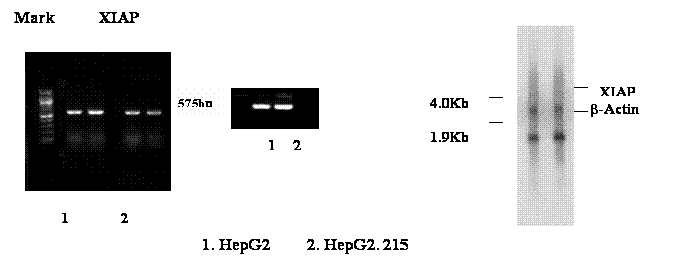
The change of another apoptosis inhibitor XIAP in the HBV expressing cell line was examined by RT-PCR and Northern blot too. The primer used to specify XIAP was IAP 3&4, which has generated a 575 base pairs band from RT-PCR (Figure 2A). The result suggests that the expression of XIAP in the HepG2.215 cells was a slightly down in the HBV expressing cell line, HepG2.215. This supports the conclusion from gene array. However, the noticeable down-regulation of XIAP in the HepG2.215 cell was not observed in the Northern blot (Figure 2B). Considering the expression of XIAP in the HepG2.215 cell was shown in the level of lower than background in the gene array (Table 1, intensity of XIAP), it is understandable that the slight decrease of XIAP in the HepG2.215 cells could be not detectable by Northern blot.
The Detection of XIAP in the HepG2.215. The RNA from HepG2.215 and HepG2 cells were isolated. The expression of XIAP was examined with RT-PCR and Northern blot as described in Figure 1. A. RT-PCR detects XIAP. B. Northern blot detects XIAP. β-Actin was used as a loading control as well.
The increase of cIAP2 protein in the HBV expressing cells
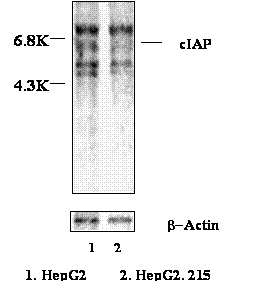
In order to further investigate the up-regulation of cIAP2 at the translation level, the HepG2.215 cells and HepG2 cells were metabolically labeled with S35 methionine. The cIAP2 then was immune-precipitated by anti-cIAP2 antibody (Zymed Laboratory, San Francisco, CA). Figure 3 shows that despite the loading of the control β-Actin has not shown difference, the cIAP2 protein with 6.8 kilo Dolton (KD) molecular weight in the HepG2.215 cells was obviously higher than that one in the HepG2 cells. This implies that the increase of cIAP2 in the HBV expressing cells was not only at the transcriptional level but also at the translational level.
The cIAP2 usually forms a heterocomplex with TNF receptor 2 (TNFR2) and its associated factors: TRAF1 and TRAF2 [31]. Therefore, those proteins might be co-precipitated with cIAP2 in our experiments. The band located beyond cIAP2 probably was the 73 KD big TNFR2. The two bands below the cIAP2 band were the 45 KD TRAF1 and 56 KD TRFA2 respectively (Figure 3).
The up-regulation of cIAP2 protein in HepG2.215 cells. HepG2.215 cells were labeled by 35-S Met. The cIAP2 was immune-precipitated by anti-cIAP2 antibody, and then resolved in SDS PAGE. The images were analyzed by Phosphorimager.
4. Discussion
The increase of expression of apoptosis inhibitor is thought to be a major cause of the tumor formation/progression. Through the inhibition of cell apoptosis, cancer cells acquire the ability of unlimited growth. The molecular mechanisms by which the IAPs exert their antiapoptotic activity have been recently revealed. The main well-characterized mechanism is the inhibition of active caspases by the direct binding of IAPs. In the many case, for example, in the lung carcinoma cells, the interaction of the over-expressed cIAP2 with caspase 9 prevents activating caspase3 that is an executer of apoptosis [10].
One of the ways to stimulate IAPs expression is virus infection. Virus infection can change the host cell gene expression, furthermore, increasing the level of IAPs as we observed in our results. Some viral protein even itself is an apoptosis inhibitor [31]. In order to determine whether HBV could stimulate IAPs expression, the cell line HepG2.215 that persistently expresses HBV and its parent cell line HepG2 that does not express HBV were compared. Despite, opposed to normal human liver cell, the abnormal level of cIAPs expression in HepG2 was observed (our unpublished data), the expression of cIAPs in HepG2.215 cells, particularly of cIAP2, were much higher than that in HepG2 cells. Because the cIAPs are the important regulators of cell apoptosis, it is possible that HBV infection might alter the cIAPs gene expressing of liver cell, finally instigating the carcinogenesis of the cells it infected. This is agreement with the observation that the patients infected by HBV have high risk to develop to hepatocellular carcinoma (HCC). However, how HBV induces alteration of cIAPs expression, if it is true, what kind of HBV protein plays role in this alteration is unknown. In an attempt, we have expressed the different HBV proteins such as surface proteins (LHBs, MHBs and SHBs), core protein and HBV X protein (HBx) in the HepG2 cells respectively. Unfortunately, we did not find any change of the apoptosis inhibitors in these cells after the transfection (unpublished data). Considering the development of HCC in HBV infected patients is a long time process, perhaps, transient transfection would not obviously alter the expression of apoptosis inhibitor at all.
Two viral peptides, the HBV X protein and the C-terminus of preS2 domain of surface protein, were found probably associating with the development of HCC [6, 13, 14, 37, 17]. However, the mechanism of how these proteins contribute to the carcinogenesis is still in the investigation. The involvement of these proteins in the NF-kB related signal transduction pathway that results in the cell apoptosis is one of the explanations.
NF-kB, a family of transcription factors, is proven to be closely connected with the cell apoptosis. However, the different NF-kB transcription factors may play diverse and even opposing roles in modulating cell death by apoptosis. The over-expression of NF-kB/RelA protects cells from tumor necrosis factor alpha (TNF-a)-or chemotherapy-mediated apoptosis [12, 32]. Enforced expression of NF-kB/RelA blocks apoptosis induced by a variety of proapoptotic agents, including TNF-a [39, 33]. In contrast, over-expression of NF-kB/c-Rel in bone marrow cells triggers apoptosis.
The role of HBx in the activation of NF-kB transcription factors is very complex. HBx can activate NF-kB signal transduction pathway, but the reaction of the cell to HBx is dependent [37]. If HBx induces NF-kB/RelA, it results in the suppression of apoptotic cell death. In contrast, if HBx induces NF-kB/c-Rel, it promotes apoptotic cell death [37]. However, how HBx triggers different cell reactions is still unknown. Interestingly, the NF-kB has been shown to up-regulate the expression of cIAP2 and cIAP1 in multiple cell lines [5, 41, 45]. There is also evidence for cIAP2 induction by NF-kB is some systems [41, 42]. Therefore, it is possible that in the HBV infected cells, HBx probably activates the NF-kB/RelA, resulting in the up-regulation of IAPs, consequently, triggering the cell apoptosis. The up-regulation of IAP by NF-kB in the HBV infected cells needs further investigation.
Using the transgenic mice, Hildt and his colleagues found that the PreS2 activators could exert a tumor-promoter-like function by activation of the PKC/c-Raf-1/MAP2-kinase signaling cascade that is a prerequisite for preS2-dependent activation of AP-1 and NF-kB [13, 14]. However, the activation of NF-kB transcription factors has been shown to correlate with the cell apoptosis, as we mentioned before, therefore, the preS2 activator perhaps also is responsible to the up-regulation of cIAPs in the HBV expressing cells.
Altogether, the development of hepatocellular carcinoma perhaps is a result of long process of multi-genetic change of the HBV infection. Nevertheless, the discovery of over-expression of cIAPs in the HBV expressing cells gives a possible way to study the relationship of cell apoptosis and the development of the carcinogenesis.
Acknowledgements
This work was supported by Nucleonics Inc, Horsham, PA, USA; Hepatitis B foundation of America and an appropriation from the Commonwealth of Pennsylvania.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Acs G, Sells MA, Purcell RH, Price P, Engle R, Shapiro M, Popper H. Hepatitis B virus produced by transfected Hep G2 cells causes hepatitis in chimpanzees. Proc Natl Acad Sci U S A. 1987 ;84(13):4641-4
2. Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997 ;3:917-921
3. Antoku K, Liu Z, Johnson DE. Inhibition of caspase proteases by CrmA enhances the resistance of human leukemic cells to multiple chemotherapeutic agents. Leukemia. 1997 ;11(10):1665-72
4. Beasley RP. The Hepatitis B virus: The major etiology of hepatocellular carcinoma. Cancer. 1988 ;61:1942-1956
5. Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci U S A. 1997 ;94(19):10057-62
6. Cromlish JA. Links Hepatitis B virus-induced hepatocellular carcinoma: possible roles for HBx. Trends Microbiol. 1996 ;4(7):270-4
7. Dai Z, Zhu WG, Morrison CD, Brena RM, Smiraglia DJ, Raval A, Wu YZ, Rush LJ, Ross P, Molina JR, Otterson GA, Plass C. A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum Mol Genet. 2003 ;12(7):791-801
8. Deveraux QL, Stennicke HR, Salvesen GS, Reed JC. Endogenous inhibitors of caspases. J Clin Immunol. 1999 ;19:388-98
9. Duckett CS, Nava VE. et al. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996 ;15:2685-2689
10. Ekedahl J, Joseph B, Grigoriev MY, Muller M, Magnusson C, Lewensohn R, Zhivotovsky B. Expression of inhibitor of apoptosis proteins in small- and non-small-cell lung carcinoma cells. Exp Cell Res. 2002 ;279(2):277-90
11. Ganem D. Assembly of hepadnaviral virions and subviral particles. Current Topics in Microbiology and Immunology. 1991 ;168:61-83
12. Harvey AJ, Soliman H, Kaiser W, Miller LK. Anti- and proapoptotic activities of baculovirus and Drosophila IAPs in an insect cell line. Cell Death Differ. 1997 ;4:733-44
13. Hildt E, Munz B, Saher G, Reifenberg K, Hofschneider PH. The PreS2 activator HBs(t) of hepatitis B virus activates c-raf-1/Erk2 signaling in transgenic mice. EMBO J. 2002 ;21(4):525-35
14. Hildt E, Hofschneider PH. The PreS2 activators of the hepatitis B virus: activators of tumor promoter pathways. Recent Results Cancer Res. 1998 ;154:315-29
15. Holcik M, Lefebvre C, Hicks K, Korneluk R. Cloning and characterization of the rat homologues of the Inhibitor of Apoptosis protein 1, 2, and 3 genes. BMC Genomics. 2002 ;3(1):5-
16. Holcik M. The IAP proteins. Trends Genet. 2002 ;18(10):537-
17. Huo TI, Wang XW, Forgues M, Wu CG, Spillare EA, Giannini C, Brechot C, Harris CC. Hepatitis B virus X mutants derived from human hepatocellular carcinoma retain the ability to abrogate p53-induced apoptosis. Oncogene. 2001 ;20(28):3620-8
18. Imoto I, Tsuda H, Hirasawa A, Miura M, Sakamoto M, Hirohashi S, Inazawa J. Expression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapy. Cancer Res. 2002 ;62(17):4860-6
19. LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. Related Articles, Links The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998 ;17(25):3247-59
20. Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda JE, MacKenzie A, Korneluk RG. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996 ;379:349-353
21. Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene. 2003 ;22:8568-80
22. Lu X, Block T. Study of the early steps of the Hepatitis B Virus life cycle. Int. J. Med. Sci. 2004 ;1(1):21-33
23. Lu X. et al. The alkylated imino sugar, n- (n-nonyl)-deoxygalactonojirimycin, reduces the amount of hepatitis B virus (HBV) nucleocapsid in tissue culture. J. Virol. 2003 ;77(22):11933-11940
24. McEleny KR, Watson RW, Coffey RN, O'Neill AJ, Fitzpatrick JM. Related Articles, Links Inhibitors of apoptosis proteins in prostate cancer cell lines. Prostate. 2002 ;51(2):133-40
25. Miller LK. An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol. 1999 ;9:323-328
26. Nachmias B, Ashhab Y, Bucholtz V, Drize O, Kadouri L, Lotem M. et al. Caspase-mediated cleavage converts Livin from an anti-apoptotic to a pro-apoptotic factor: implications for drugresistant melanoma. Cancer Res. 2003 ;63:6340-9
27. Ogden SK, Lee KC, Barton MC. Hepatitis B viral transactivator HBx alleviates p53-mediated repression of alpha-fetoprotein gene expression. J Biol Chem. 2000 ;275(36):27806-14
28. Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R, Gabbert H, Gerharz C. XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Human pathology. 2004 ;35(8):1022-1028
29. Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr. Opin. Oncol. 1999 ;11:68-75
30. Reed JC. The Survivin saga goes in vivo. J. Clin. Investig. 2001 ;108:965-969
31. Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995 ;83:1243-52
32. Schulze-Bergkamen H, Krammer PH. Apoptosis in cancer implications for therapy. Semin Oncol. 2004 ;31:90-119
33. Schultz IJ, Kiemeney LA, Witjes JA, Schalken JA, Willems JL, Swinkels DW. Survivin mRNA expression is elevated in malignant urothelial cell carcinomas and predicts time to recurrence. anti-cancer Res. 2003 ;23:3327-31
34. Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A. 1987 ;84(4):1005-9
35. Sells MA, Zelent AZ, Shvartsman M, Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988 ;62(8):2836-44
36. Srikanth S, Kraft AS. Inhibition of caspases by cytokine response modifier A blocks androgen ablation-mediated prostate cancer cell death in vivo. Cancer Res. 1998 ;58(4):834-9
37. Su F, Theodosis CN, Schneider RJ. Role of NF-kappaB and myc proteins in apoptosis induced by hepatitis B virus HBx protein. J Virol. 2001 ;75(1):215-25
38. Summers J, Mason W S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982 ;29:403-415
39. Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA. 2001 ;98:8662-7
40. Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S. et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000 ;6:1796-803
41. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998 ;281(5383):1680-3
42. Wen L, Zhuang L, Luo X, Wei P. TL1A-induced NF-kappaB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem. 2003 ;278(40):39251-8
43. Young SS, Liston P, Xuan JY, McRoberts C, Lefebvre CA, Korneluk RG. Genomic organization and physical map of the human inhibitors of apoptosis: HIAP1 and HIAP2. Mamm Genome. 1999 ;10:44-48
44. Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003 ;63(20):6815-24
45. Zou T, Rao JN, Guo X, Liu L, Zhang HM, Strauch ED, Bass BL, Wang JY. NF-kappaB-mediated IAP expression induces resistance of intestinal epithelial cells to apoptosis after polyamine depletion. Am J Physiol Cell Physiol. 2004 ;286(5):C1009-18
Author biography
Xuanyong Lu, PhD is an Assistant Professor of Drexel Institute for Biotechnology and Virology Research, School of Medicine, Drexel University, USA. His current researches investigate the entry of hepatitis B virus, pathology of HBV infection and hepatocellular carcinoma (HCC). Previously he was an assistant professor in Department of Biochemistry and Molecular Pharmacology, Thomas Jefferson University, Philadelphia, USA. Dr. Lu is the Chief Scientist of Hepatitis B Foundation of America.
Matthew Lee (Intel Scientific Award winner) currently is in the New York University. He is involved in the investigation of Apoptosis inhibitor expression in the hepatoma cells.
Trang Tran (BS) is formal Research Assistant in the Drexel Institute for Biotechnology and Virology Research, Drexel University, and is now pursuing her graduate degree at Medical College of Thomas Jefferson University, USA. She is involved in the investigation of the role of HBV proteins in the up-regulation of apoptosis inhibitor.
Timothy Block Ph. D is a Professor, director of Drexel Institute for Biotechnology and Virology Research, School of Medicine, Drexel University. His current researches include the role of glycan in the HBV infection, assembly and secretion, the anti-HBV drug exploration and development and the latent of Herpes simple virus. Prof. Block is the director of laboratory of Hepatitis B Foundation of America.
![]() Corresponding address:
Corresponding address:
Xuanyong Lu, Drexel Institute for Biotechnology and Virology Research, Department of Microbiology and Immunology, School of Medicine, Drexel University. 700 East Butler Ave., Doylestown, PA, 18901 USA. Telephone: 215-489-4906. Fax: 215-489-4920. E-mail: Xuanyong.luedu.

 Global reach, higher impact
Global reach, higher impact