ISSN: 1449-1907International Journal of Medical Sciences
Int J Med Sci 2017; 14(13):1430-1435. doi:10.7150/ijms.22294 This issue Cite
Research Paper
ZW800-1 for Assessment of Blood-Brain Barrier Disruption in a Photothrombotic Stroke Model
1. Department of Otolaryngology-Head and Neck Surgery, Chonnam National University Medical School, Gwangju 61469, South Korea;
2. Department of Premedical Program, School of Medicine, Chosun University, Gwangju 61452, South Korea;
3. Department of Forensic Medicine, Chonnam National University Medical School, Gwangju 61469, South Korea;
4. Department of Biomedical Sciences, Chonnam National University Medical School, Gwangju 61469, South Korea;
5. Department of Nuclear Medicine, Chonnam National University Medical School, Gwangju 61469, South Korea.
* These authors contributed equally to this work.
Abstract
Background: Since it is known that serum albumin-bound dyes can cross the blood-brain barrier (BBB) after ischemia, Evans Blue dye is commonly used to assess BBB disruption because of its rapid binding to serum albumin. In addition, indocyanine green (ICG), a clinically available dye, binds to serum proteins that could also be used for assessment of BBB impairment. Unlike these near-infrared (NIR) dyes, zwitterionic NIR fluorophore (ZW800-1) shows no serum binding, ultralow non-specific tissue uptake, and rapid elimination from the body via renal filtration. In this study, we report the use of ZW800-1 as a NIR fluorescence imaging agent for detecting BBB disruption in rat stroke models.
Methods: Three types of NIR fluorophores, Evans Blue, ICG, and ZW800-1, were administered intraperitoneally into rat photothrombotic stroke models by using 4% concentration of each NIR dye. The NIR fluorescence signals in the infarcted brain tissue and biodistribution were observed in real-time using the Mini-FLARE® imaging system up to 24 h post-injection.
Results: ZW800-1 provided successful visualization of the ischemic injury site in the brain tissue, while the remaining injected dye was clearly excreted from the body within a certain period of time. Although Evans Blue and ICG provided mapping of the infarcted brain lesions, they exhibited high non-specific uptake in most of the tissues and organs and persisted in the body over 24 h post-injection.
Conclusion: Our results suggest the promising application of ZW800-1 as a new strategy in BBB experiments and future therapeutic development.
Keywords: ZW800-1, photothrombotic stroke model, blood-brain barrier, near-infrared fluorescence.
Introduction
Blood-brain barrier (BBB) is characterized by highly selective permeability between blood and brain parenchyma. It is well known that this property is important for protecting the central nervous system from many potential toxic products, whilst effectively exchanging solutes and fluid for normal brain metabolism. Various types of brain disorders such as ischemia, trauma, inflammation, neurodegenerative disease, and epilepsy are associated with BBB destruction, leading to further degradation of neuronal and synaptic functions [1, 2]. The important role of the BBB was actually revealed and established with the development of the assessment techniques. Starting from the early 20th century in which trypan blue was used, many molecules have been used to visualize the integrity of the BBB [3]. However, no molecule seems to perfectly satisfy the criteria of both effectiveness and safety for this task.
Molecules used for BBB analysis usually share their properties with those of 'non-BBB permeable tracers'. Fluorescein, Evans blue, Horseradish peroxidase (HRP), Sucrose, Dextran, Radio-iodinated albumin, Radio-iodinated inulin, IgG, Trypan blue, and Fibrinogen are used as markers for evaluating the BBB status [4]. Currently, Evans Blue dye is the most commonly used molecule by injecting 2-4% concentration. Evans Blue is known to bind to serum albumin, and thus, it cannot cross the BBB in normal conditions [4]. When the BBB is disrupted, the dye can leak into the brain parenchyma and can be detected by the naked eye. Although Evans Blue was used in many BBB researches, this molecule can cause severe adverse effects such as pulmonary embolism or toxic metabolic encephalopathy, especially in higher concentrations [4]. Moreover, it can also bind to tissue protein and can get accumulated randomly in the body. Thus, it cannot be applied in clinical trials. Other molecules have drawbacks such as high cost, very rapid clearance, low signal-to-background ratio, and unreliable quantification issues.
Near-infrared (NIR; 650-900 nm) fluorescence imaging has emerged as a non-invasive, non-ionizing and real-time visualization technique. Compared to conventional fluorescent dyes, these dyes show ultralow autofluorescence providing high signal-to-background ratio images [5]. Furthermore, NIR fluorophores can be manipulated to achieve a conformational change in order to acquire various properties such as tissue-specific binding, rapid excretion from the body, or other therapeutic purposes [6-10]. However, only few studies have been conducted to assess the feasibility of NIR fluorophores for the BBB evaluation [11-13]. Indocyanine green (ICG) is a clinically available NIR fluorophore; however, it is far from optimal since it shows high uptake in the liver and gastrointestinal tract, and it binds to serum proteins that could also be used for assessment of BBB impairment. Unlike these near-infrared (NIR) dyes, zwitterionic NIR fluorophore (ZW800-1) shows no serum binding, ultralow non-specific tissue uptake, and rapid elimination from the body via renal filtration. [14-16]. Herein, we report the use of ZW800-1 as a NIR fluorescence imaging agent for detecting BBB disruption in rat stroke models.
Materials and Methods
NIR fluorophores and animals
Evans Blue dye and ICG were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. ZW800-1 was prepared as described previously [14,15]. Animal care, experiments, and euthanasia were performed in accordance with protocols approved by the Chonnam National University Animal Research Committee. Adult (8-week-old) male Sprague-Dawley (SD) rats weighing ≈ 250 g (N = 5, independent experiments) were purchased from Samtako (Seoul, South Korea).
Establishment of a photothrombotic stroke model
Focal cortical ischemia was induced by photothrombosis of cortical microvessels using Rose Bengal (Sigma-Aldrich, St. Louis, MO, USA) with cold light (Zeiss KL1500 LCD, Germany) [17]. Each animal was anesthetized with 5% isoflurane and anesthesia was maintained with 3% isoflurane in an oxygen/air mixture using a gas anesthesia mask in a stereotaxic frame (Stoelting, Wood Dale, IL, USA). Body temperature was maintained at 37 ± 0.5°C during surgery using a heating pad controlled by a rectal probe. For illumination, a 4.5 mm fiber-optic bundle from a cold light source was positioned on the exposed skull 0.5 mm anterior to the bregma and 3.7 mm lateral to the midline over the left sensorimotor cortex, as previously described [18]. The brain was illuminated for 10 minutes after infusion of 50 mg/kg Rose Bengal in normal saline into the right femoral vein via a microinjection pump within 1 minute. The scalp was sutured, and the mice were allowed to wake up before being returned to their home cages.
NIR fluorescence imaging system
In vivo NIR fluorescence imaging was performed using the Mini-FLARE® imaging system as described previously [8]. Briefly, the system consists of three separate light sources having different wavelengths: a “white” LED light source, generating 26,600 lux of 400-650 nm light to illuminate the surgical field, and NIR LED light sources, generating Channel #1 (656-678 nm excitation; 689-725 nm emission; 1.08 mW/cm2 fluence rate) and Channel #2 (745-779 nm excitation; 800-848 nm emission; 7.70 mW/cm2 fluence rate). White light and NIR fluorescence images were acquired simultaneously and displayed in real-time, using custom-designed optics and software.
Intraoperative fluorescence imaging of BBB disruption and biodistribution in animal models of stroke
For intraoperative NIR fluorescence imaging, dye stock solutions were dissolved in PBS from 10 mM concentrations and administered intraperitoneally into rat stroke models by using 4% concentration of each NIR dye. The NIR fluorescence signals in the infarcted brain tissue and biodistribution were observed in real-time using the Mini-FLARE® imaging system up to 24 h post-injection.
Results
Physicochemical and optical properties of Evans Blue, ICG and ZW800-1 NIR fluorophores
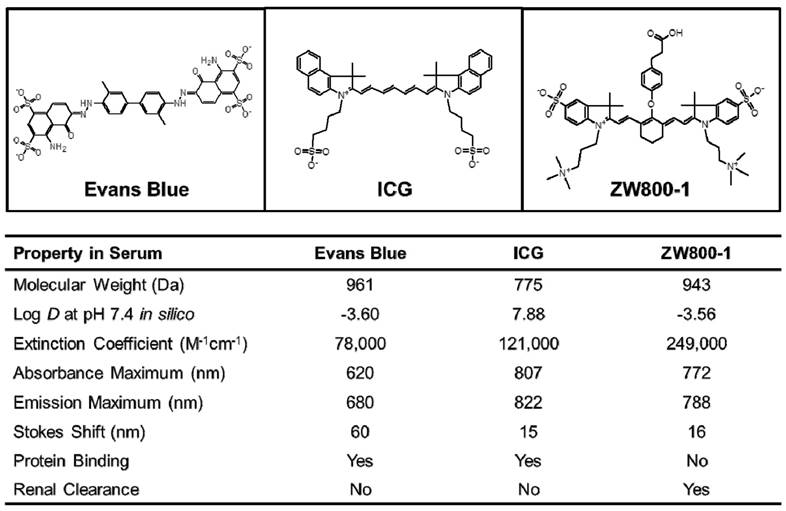
The physicochemical and optical properties of Evans Blue, ICG, and ZW800-1 NIR fluorophores are detailed in Figure 1. Of particular importance is the geometrical balance of charge (net zero) over each fluorophore's surface, which stands in contrast to the most widely used Evans Blue, whose sulfonated form has a net negative charge. Log D value at pH 7.4 for ZW800-1 determined in silico was -3.56, which was highly hydrophilic compared to the clinically available ICG (7.88), but the log D value of ZW800-1 was similar to that of Evans Blue (-3.60). ZW800-1 also exhibited a relatively higher molar extinction coefficient (ε) in serum compared to Evans Blue (ε = 78,000 M-1cm-1) and ICG (ε = 121,000 M-1cm-1). Of note, Evans Blue and ICG showed significant serum protein binding due to the net negative charge of Evans Blue and the hydrophobicity of ICG. However, ZW800-1 with high hydrophilicity and balanced net charge distributed evenly over its surface did not absorb serum proteins. Elimination of the NIR fluorophores from the body via renal clearance also varied, with Evans Blue and ICG showing hepatobiliary clearance and ZW800-1 being mostly renally cleared [4,14-16,19,20].
Mapping of infarcted brain lesions by using Evans Blue, ICG and ZW800-1 NIR fluorophores in animal models of stroke
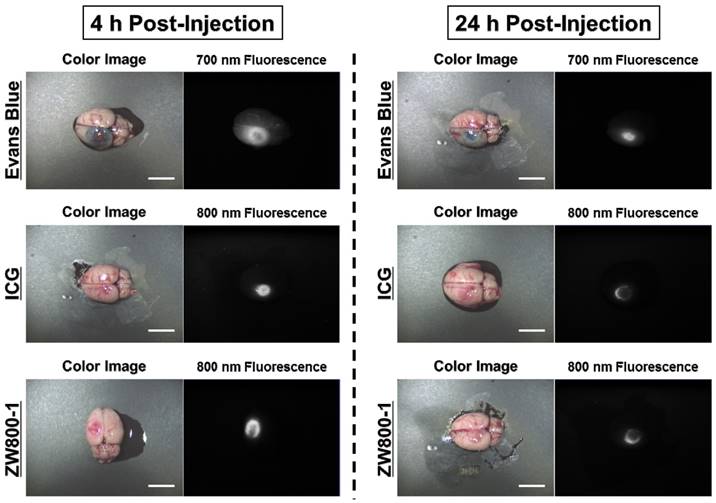
By using the conventional dose of Evans Blue dye, 4% concentrations of Evans Blue, ICG, and ZW800-1 were intraperitoneally injected into rat photothrombotic stroke models. After 4 h injection, Evans Blue stained the ischemic site in the brain tissue and it was possible to identify the infarcted lesion with the naked eye as a blue color. ICG was also clearly detected at the ischemic site and the fluorescence signal in the infarcted brain lesion was significantly decreased within 24 h post-injection. Importantly, ZW800-1 showed strong fluorescence signal in the ischemic stroke area at 4 h post-injection, which was similar to other dyes. Although the fluorescence signal gradually decreased, it still persisted over 24 h post-injection. This result indicates that ZW800-1 could be used for detecting BBB disruption in rat stroke models within a certain period of time (Figure 2).
Chemical structures and physicochemical and optical properties of Evans Blue, ICG and ZW800-1 NIR fluorophores in 100% serum, pH 7.4 [14-16, 19, 20]. In silico calculations of the Log D value at pH 7.4 were calculated using Marvin and JChem calculator plugins (ChemAxon, Budapest, Hungary).
In vivo NIR imaging of the infarcted brain tissue using Evans Blue, ICG and ZW800-1 in rat stroke models. 4% concentration of each dye was injected intraperitoneally into 250 g SD-rats 24 h prior to imaging and resection. Scale bars = 1 cm. Images are representative of N = 5 independent experiments. All NIR fluorescence images have identical exposure and normalizations.
In vivo biodistribution and excretion of Evans Blue, ICG and ZW800-1 NIR fluorophores
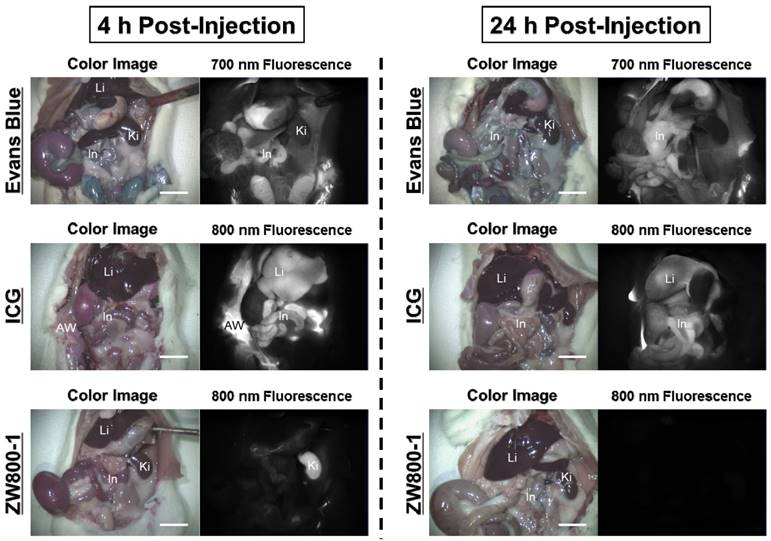
Intraperitoneal injections of 4% Evans Blue, ICG, and ZW800-1 dissolved in PBS were individually administered to rat stroke models 24 h prior to imaging. As expected, Evans Blue showed blue-colored staining in most of the organs with highest contamination in the abdomen. ICG also exhibited high uptake in the liver and contaminated the gastrointestinal tract as well as the abdominal wall. The fluorescence signals of Evans Blue and ICG still persisted in the body over 24 h post-injection. Unlike Evans Blue and ICG, ZW800-1 was exclusively cleared by the kidneys, with no appreciable non-specific background signal in any tissues and organs at 4 h post-injection and was completely excreted from the body within 24 h post-injection (Figure 3).
Discussion
Modalities for evaluating the BBB can be divided into invasive and non-invasive techniques [21]. The invasive technique needs brain harvesting and requires further tissue processing. Although this can be a quantitative method, it is rather useful for research purpose only and cannot be applied for clinical use.
Among the non-invasive in vivo methods, medical imaging techniques such as CT, MRI, PET, and SPECT are already being used in the clinic. MRI with gadolinium contrast imaging has been reported [22]. High resolution MR imaging can also provide more spatial information. However, it needs a delicate comparison of the signal change and shows inferiority in quantification. PET can be applied to determine whether the cellular transport mechanism of the BBB is intact. 18F-2-fluoro-2-deoxy-D-glucose (18F-FDG) or 2'methoxyphenyl-(N-2'-pyridinyl)-p-fluoro-benzamidoethylpiperazine ([18F]MPPF) were used for this task [23,24]. However, this is not a direct method for determining BBB permeability impairment. Direct optical imaging can be performed after making a bony window and by injecting a specific fluorescence dye, Lucifer yellow CH dipotassium salt [25]. Semi-quantitative analysis is also possible by using this method; however, a craniotomy is needed for the study. Status of the BBB can also be checked by an indirect method, by using biomarkers. S100ß is a brain tissue protein which is normally not found in the blood circulation. After BBB disruption, it was detected in the serum by ELISA method [26]. However, this method cannot provide visualization of the site of BBB breakdown.
In vivo biodistribution of Evans Blue, ICG and ZW800-1 in rats. 4% concentration of each dye was injected intraperitoneally into 250 g SD-rats 24 h prior to imaging and resection. Abbreviations used are: AW, abdominal wall; In, intestine; Ki, kidneys; Li, liver. Scale bars = 1 cm. Images are representative of N = 5 independent experiments. All NIR fluorescence images have identical exposure and normalizations.
Currently, NIR fluorescence imaging seems to be somewhat between preclinical and clinical use. It is actually a subtype of fluorescence imaging in which a specific wavelength of 650-900 nm is used. It is an emerging technique due to ultralow autofluorescence and excellent signal-to-background ratio. It is widely used in an in vivo animal study for intraoperative image-guided surgery, and ICG has already been used in cancer surgery to detect metastatic lesions [27]. However, only limited number of studies has tried to assess the feasibility of NIR fluorophores for BBB evaluation.
In the present study, we found that a novel NIR dye, ZW800-1, can be used as an alternative method for detecting the BBB impairment site after injury. ZW800-1 was designed to have a net change of 0. Since charges of a molecule provide more affinity to other molecules, one might expect that ZW800-1 will be free from binding to other components of the tissue. In fact, it was excreted from the body within 4 h after systemic administration [14]. Importantly, ZW800-1 stained the ischemic site of brain tissue at 4 h post-injection, while the remaining injected dye was clearly excreted from the body within a certain period of time.
The important issue related to BBB exploration is the tracer molecule. The following properties of an 'ideal' molecule for evaluating the BBB have been suggested:(1) it should be metabolically inert, (2) it should be non-toxic, (3) it should be non-binding to plasma or tissue proteins, (4) it should be visualized easily, and (5) it should provide reliable quantification [4]. Evans Blue is the most popularly used tracer in BBB research. It cannot penetrate the intact BBB before binding to serum albumin. When the BBB is destroyed, it extravasates and can be easily identified by its blue color. Quantification was also performed by brain extraction and spectrophotometry. However, it is now known that when the concentration of Evans Blue exceeds the binding capacity of albumin, some amount of Evans Blue can be in the free form [28]. Albumin free-Evans Blue can enter several tissues and bind to their proteins. This can hinder the reliability of the quantification. Moreover, it can accumulate in the tissue and cause toxic effects. Therefore, ZW800-1 could be used to overcome this problem, because it exists solely in the body and shows no serum binding and ultralow non-specific tissue uptake. This property makes it closer to an 'ideal' tracer for BBB evaluation, even superior to the other NIR dyes like ICG or albumin-NIR conjugate. The exact mechanism by which ZW800-1 persists at the ischemic site is still unclear. The circulation might have slowed down in the BBB impaired area.
In conclusion, ZW800-1 NIR fluorophore provides excellent visualization of the ischemic injury site, while it was clearly excreted from the body within a certain period of time. It is an efficient new method for assessing BBB breakdown without causing any toxicity. ZW800-1 can be a new strategy in BBB experiments and future therapeutic development.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grant (no. NRF-2015R1C1A1A01053168; H.H., NRF-2016R1A2B4008316; K.H.S., and NRF-2017R1D1A1A02018640; S.L.) and the Pioneer Research Center Program (2015M3C1A3056410; H.H.) funded by the Ministry of Science, ICT and Future Planning (MSIP), Republic of Korea.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178-201
2. Alimohammadi H, Fakhri S, Derakhshanfar S, Hosseini-Zijoud SM, Safari S, Hatamabadi HR. The effects of air pollution on ischemic stroke admission rate. Chonnam Med J. 2016;52(1):53-8
3. Saunders NR, Dreifuss JJ, Dziegielewska KM, Johansson PA, Habgood MD, Mollgard K. et al. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Frontiers in neuroscience. 2014;8:404
4. Saunders NR, Dziegielewska KM, Mollgard K, Habgood MD. Markers for blood-brain barrier integrity: how appropriate is Evans blue in the twenty-first century and what are the alternatives?. Frontiers in neuroscience. 2015;9:385
5. Jo D, Hyun H. Structure-inherent targeting of near-infrared fluorophores for image-guided surgery. Chonnam Med J. 2017;53(2):95-102
6. Lim W, Sohn H, Ko Y, Park M, Kim B, Jo D. et al. Real-time in vivo imaging of metastatic bone tumors with a targeted near-infrared fluorophore. Oncotarget. 2017;8(39):65770-7
7. Owens EA, Hyun H, Dost TL, Lee JH, Park G, Pham DH. et al. Near-infrared illumination of native tissues for image-guided surgery. J Med Chem. 2016;59(11):5311-23
8. Hyun H, Park MH, Owens EA, Wada H, Henary M, Handgraaf HJ. et al. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat Med. 2015;21(2):192-7
9. Hyun H, Owens EA, Wada H, Levitz A, Park G, Park MH. et al. Cartilage-specific near-infrared fluorophores for biomedical imaging. Angew Chem Int Ed. 2015;54(30):8648-52
10. Hyun H, Wada H, Bao K, Gravier J, Yadav Y, Laramie M. et al. Phosphonated near-infrared fluorophores for biomedical imaging of bone. Angew Chem Int Ed. 2014;53(40):10668-72
11. Ergin A, Wang M, Zhang JY, Bruce JN, Fine RL, Bigio IJ. et al. The feasibility of real-time in vivo optical detection of blood-brain barrier disruption with indocyanine green. J Neurooncol. 2012;106(3):551-60
12. Li S, Johnson J, Peck A, Xie Q. Near infrared fluorescent imaging of brain tumor with IR780 dye incorporated phospholipid nanoparticles. J Transl Med. 2017;15(1):18
13. Klohs J, Steinbrink J, Bourayou R, Mueller S, Cordell R, Licha K. et al. Near-infrared fluorescence imaging with fluorescently labeled albumin: a novel method for non-invasive optical imaging of blood-brain barrier impairment after focal cerebral ischemia in mice. J Neurosci Methods. 2009;180(1):126-32
14. Choi HS, Nasr K, Alyabyev S, Feith D, Lee JH, Kim SH. et al. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew Chem Int Ed. 2011;50(28):6258-63
15. Hyun H, Bordo MW, Nasr K, Feith D, Lee JH, Kim SH. et al. cGMP-Compatible preparative scale synthesis of near-infrared fluorophores. Contrast Media Mol Imaging. 2012;7(6):516-24
16. Hyun H, Henary M, Gao T, Narayana L, Owens EA, Lee JH. et al. 700-nm zwitterionic near-infrared fluorophores for dual-channel image-guided surgery. Mol Imaging Biol. 2016;18(1):52-61
17. Choi KH, Kim HS, Park MS, Kim JT, Kim JH, Cho KA. et al. Regulation of caveolin-1 expression determines early brain edema after experimental focal cerebral ischemia. Stroke. 2016;47(5):1336-43
18. Wiessner C, Bareyre FM, Allegrini PR, Mir AK, Frentzel S, Zurini M. et al. Anti-Nogo-A antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2003;23(2):154-65
19. Wang Y, Lang L, Huang P, Wang Z, Jacobson O, Kiesewetter DO. et al. In vivo albumin labeling and lymphatic imaging. Proc Natl Acad Sci USA. 2015;112(1):208-13
20. Berezin MY. Nanotechnology for Biomedical Imaging and Diagnostics: From Nanoparticle Design to Clinical Applications. Wiley Interscience: New York. 2014 pp:312-313
21. Chassidim Y, Vazana U, Prager O, Veksler R, Bar-Klein G, Schoknecht K. et al. Analyzing the blood-brain barrier: the benefits of medical imaging in research and clinical practice. Semin Cell Dev Biol. 2015;38:43-52
22. Chassidim Y, Veksler R, Lublinsky S, Pell GS, Friedman A, Shelef I. Quantitative imaging assessment of blood-brain barrier permeability in humans. Fluids Barriers CNS. 2013;10(1):9
23. Wunder A, Klohs J, Dirnagl U. Non-invasive visualization of CNS inflammation with nuclear and optical imaging. Neuroscience. 2009;158(3):1161-73
24. Bartmann H, Fuest C, la Fougere C, Xiong G, Just T, Schlichtiger J. et al. Imaging of P-glycoprotein-mediated pharmacoresistance in the hippocampus: proof-of-concept in a chronic rat model of temporal lobe epilepsy. Epilepsia. 2010;51(9):1780-90
25. Prager O, Chassidim Y, Klein C, Levi H, Shelef I, Friedman A. Dynamic in vivo imaging of cerebral blood flow and blood-brain barrier permeability. Neuroimage. 2010;49(1):337-44
26. Marchi N, Cavaglia M, Fazio V, Bhudia S, Hallene K, Janigro D. Peripheral markers of blood-brain barrier damage. Clin Chim Acta. 2004;342(1-2):1-12
27. Namikawa T, Sato T, Hanazaki K. Recent advances in near-infrared fluorescence-guided imaging surgery using indocyanine green. Surg Today. 2015;45(12):1467-74
28. Allen TH, Orahovats PD. Combination of toluidine dye isomers with plasma albumin. Am J Physiol. 1950;161(3):473-82
Author contact
![]() Corresponding authors: Hoon Hyun, Ph.D., 160 Baekseo-ro, Dong-gu, Gwangju 61469, South Korea Office: +82-61-379-2652; Fax: +82-61-379-8455 Email: hhyunac.kr Hyung-Seok Kim, M.D., Ph.D., 160 Baekseo-ro, Dong-gu, Gwangju 61469, South Korea Office: +82-62-220-4092; Fax: +82-62-223-4250 Email: veritasac.kr
Corresponding authors: Hoon Hyun, Ph.D., 160 Baekseo-ro, Dong-gu, Gwangju 61469, South Korea Office: +82-61-379-2652; Fax: +82-61-379-8455 Email: hhyunac.kr Hyung-Seok Kim, M.D., Ph.D., 160 Baekseo-ro, Dong-gu, Gwangju 61469, South Korea Office: +82-62-220-4092; Fax: +82-62-223-4250 Email: veritasac.kr
Received 2017-8-9
Accepted 2017-10-13
Published 2017-11-2
