3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(1):43-49. doi:10.7150/ijms.5358 This issue Cite
Research Paper
Peginterferon Alfa-2a plus Ribavirin in Japanese Patients Infected with Hepatitis C Virus Genotype 2 Who Failed Previous Interferon Therapy
1. Department of Gastroenterology and Nephrology, Graduate School of Medicine, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba 260-8677, Japan.
2. Department of Gastroenterology, Tokyo Women's Medical University Yachiyo Medical Center, Yachiyo 276-8524, Japan.
3. Department of Gastroenterology, Toho University Sakura Medical Center, Sakura 285-8741, Japan.
4. Institute of Clinical Medicine and Research, Jikei University School of Medicine, Kashiwa 277-8567, Japan.
5. Department of Gastroenterology, Narita Red Cross Hospital, Narita 286-8523, Japan.
6. Department of Medicine, Kikkoman Hospital, Noda 270-0116, Japan.
Received 2012-10-10; Accepted 2012-12-2; Published 2012-12-10
Abstract
Some patients infected with hepatitis C virus (HCV) genotype 2 could be cured with treatment shorter than 24 weeks using peginterferon plus ribavirin, but there are still treatment-refractory patients. Direct-acting antivirals (DAAs) are not currently available for HCV genotype 2 patients, different from genotype 1 patients, in clinical practice. We investigated 29 HCV genotype 2-infected Japanese patients who had been previously treated and failed to clear HCV. We retreated them with peginterferon alfa-2a plus ribavirin and measured HCV RNA level to assess the efficacy and safety of this treatment in patients who had failed previous therapy. We found that retreatment of HCV genotype 2-infected Japanese patients with peginterferon alfa-2a plus ribavirin for 24-48 weeks led to 60 to 66.6% sustained virological response (SVR) in patients previously treated with (peg-)interferon monotherapy and to 69.9% SVR in relapsers previously treated with peginterferon plus ribavirin. Attention should be paid to certain patients with unique features. Selection of patients according to their previous treatment could lead to optimal therapy in HCV genotype 2 treatment-experienced patients.
Keywords: Retreatment, HCV G2, Japanese
INTRODUCTION
Hepatitis C virus (HCV) infection causes acute and chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC) [1]. HCV is also a major causative agent of HCC in Japan [2]. HCV is a positive-sense single stranded RNA virus with ~9.6 kb length, belonging to the genus Hepacivirus, a member of the family Flaviviridae. It is known that there exist at least 6 main genotypes of HCV [3]. These approximately equidistant genetic groups each contain a variable number of more closely related, genetically and epidemiologically distinct “subtypes”. Genotypes differ from each other by 31 to 33% at the nucleotide level, compared with 20 to 25% between subtypes [3]. In Japan, HCV genotype 1b, 2a and 2b, respectively, are observed in ~70, 20 and 10% of HCV-infected patients [4].
Treatment with peginterferon plus ribavirin for 24 weeks leads to 70-80% sustained virological response (SVR) in treatment-naïve patients infected with HCV genotype 2 [5-7]. Combination of peginterferon with ribavirin for 24 weeks is the current standard of care (SOC) for treatment-naïve patients infected with HCV genotype 2 or 3. Some selected HCV genotype 2-infected patients achieved SVR with treatment periods shorter than 24 weeks [5-9]. However, in treatment-naïve patients infected with HCV genotype 1, treatment with peginterferon plus ribavirin for 48 weeks leads to only ~50% SVR [7]. Thus, HCV genotype is one of the important factors influencing the outcome of interferon treatments [7,10].
Retreatment of chronic hepatitis C patients failing to achieve SVR with combination peginterferon plus ribavirin could only obtain 10 to 15% SVR in non-responders and 40 to 50% SVR in relapsers [11]. In North America and European countries, retreatment for HCV genotype 2 or 3 patients failing to achieve SVR with combination peginterferon plus ribavirin could lead to 37 to 46% SVR in non-responders and 52 to 63% in relapsers [12, 13], even though retreatment with SOC was performed for 48 weeks. In our previous study [14], we observed that retreatment for HCV genotype 2 Japanese patients who failed to achieve SVR with combination peginterferon alfa-2b plus ribavirin for 16, 24 or 48 weeks resulted in 71.4% SVR, but the proportions of non-responders and relapsers were unclear and HCV RNA was measured with COBAS AMPLICOR HCV Monitor Test v. 2.0 (range: 0.5 - 850 kIU/mL) (Roche Diagnostics, Tokyo, Japan).
In the present study, we investigated 29 HCV genotype 2-infected Japanese patients who had been previously treated and failed to clear HCV. We retreated them with peginterferon alfa-2a plus ribavirin and measured HCV RNA with the more sensitive COBAS TaqMan HCV test (Roche) to assess the efficacy and safety of peginterferon alfa-2a with ribavirin in patients who had failed previous therapy in clinical practice. We focused on 3 females retreated with peginterferon alfa plus ribavirin and resulting in non-SVR, in whose sera a single very low-titer fluctuation of HCV RNA from negative to positive was detected after HCV RNA had been undetected. This would indicate that treatment with SOC should be stopped in HCV genotype 2 female patients with these features.
MATERIALS AND METHODS
Patients
Patients were recruited from Chiba University Hospital and 29 hospitals in Chiba, Ibaraki, and Saitama Prefectures between March 2008 and September 2011. Patients were eligible if they met the following inclusion criteria: (i) infected with HCV genotype 2 alone, (ii) age ≥ 20 years, (iii) diagnosed as chronic hepatitis C, (iv) negative for HBs antigen, (V) negative for human immunodeficiency virus, (vi) no autoimmune liver diseases, (vii) no severe renal disease, (viii) no severe heart disease, (ix) no mental disorders, (x) no current intravenous drug abuse, and (xi) no pregnancy. Thirty-four of the patients had previously been included in an investigation of the incidence of HCC during and immediately after peginterferon alfa-2a and ribavirin treatment in patients with chronic hepatitis C in Japan [2].
Study design
We recruited previously treated patients infected with HCV genotype 2. In Japan, combination therapy for treatment-naïve patients infected with HCV genotype 2 was not supported by the Japanese health insurance system at that time [15]. Concerning previously treated patients, they had to have failed previous treatment with either conventional interferon monotherapy, peginterferon monotherapy, conventional interferon/ribavirin combination therapy, or peginterferon/ribavirin combination therapy, different from the previous study by Sherman et al. [12]. Twenty-nine consecutive patients were enrolled in this study. Informed consent was obtained from all patients prior to enrolment. The Ethics Committee of Chiba University School of Medicine approved the study protocol. In this study, 180 μg of peginterferon alfa-2a per week plus 600-800 mg ribavirin per day were usually given in the treatment of patients for as long as 24, 48, or 72 weeks, according to the patient's will, as combination therapy for retreated patients infected with HCV genotype 2 was supported for only 24 weeks by the Japanese health insurance system at that time [15]. Clinical and laboratory assessments were performed at least every 4 weeks during treatment and a 12-week follow-up period. Adverse events were noted by oral inquiry (patient interview), physical examinations and laboratory tests.
Determination of HCV RNA titers and HCV genotype
Serum HCV RNA titer was measured using COBAS TaqMan HCV test (Roche), with levels ranging from 1.2 to 7.8 log IU/mL [16]. HCV genotype was determined by the antibody serotyping method of Tukiyama-Kohara et al. [17,18]. According to this assay, HCV serotypes 1 and 2 correspond to HCV genotypes 1a/1b and 2a/2b [3]. HCV RNA titer and HCV genotype were determined before treatment, and HCV RNA was measured every 4 weeks before, during, and for at least 24 weeks after the end of treatment.
Serum liver function tests and hematology tests
Serum aminotransferase concentrations, other liver function tests and hematology tests were performed according to standard methods every 1 to 3 months before, during, and for at least 24 weeks after the end of treatment.
Assessment of efficacy
SVR was defined as undetectable serum HCV RNA at 24 weeks after the end of treatment. Relapse was defined as undetectable HCV RNA at the end of therapy, followed by the reappearance of HCV RNA [11]. Non-response was defined as detectable HCV RNA at the end of therapy. Patients with undetectable HCV RNA within the initial 4 weeks of treatment were considered to have had rapid virological response (RVR). Patients who had undetectable HCV RNA within the initial 12 weeks of treatment were considered to have had complete early virological response (cEVR) (described as EVR in this article) [16].
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Differences were evaluated by Student's t-test, chi-square test, or Fisher's exact test. P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
The characteristics of the 29 patients in the present study are shown in Table 1. They had a history of peginterferon/conventional interferon with or without ribavirin, and 4 were unknown regarding previous treatment response (Table 1). In these 29 patients, 3 received conventional interferon monotherapy, 10 peginterferon alfa-2a monotherapy, 12 peginterferon alpha-2b plus ribavirin, 3 peginterferon alfa-2a plus ribavirin, and 1 had details unknown. HCV RNA levels (≥5log IU/mL, <5log IU/mL, and unknown) were 24, 4 and 1, respectively. Concerning virological response, 18 (62.0%) had SVR, 9 (31.0%) relapsed, and 2 (6.8%) discontinued treatment due to side effects.
Baseline and demographic characteristics of patients in the present study
| Number of patients | 29 |
| Age (years) | 60.1 ± 8.6 |
| Gender (male/female) | 15/14 |
| Body mass index (kg/m2) | 26.2 ± 3.6 |
| HCV RNA (log IU/mL) | 5.5 ± 2.0 |
| ALT (IU/L) | 57.8 ± 50.7 |
| γ-GTP (IU/L) | 46.0 ± 40.7 |
| AFP (ng/mL) | 5.7 ± 3.4 |
| Leukocyte count (/mm3) | 4940 ± 1670 |
| Hemoglobin (g/dL) | 14.0 ± 1.6 |
| Platelet count (x104/mm3) | 16.2 ± 5.2 |
| Treatment response | |
| Duration of treatment (~24/48/72 wks) | 9/18/2 |
| RVR rates, % | 34.4 (10/29) |
| HCV RNA negativity at 8 wks | 81.4 (22/27) |
| EVR rates, % | 88.8 (24/27) |
| SVR rates, % | 62.0 (18/29) |
Data are expressed as mean ± SD. ALT, alanine aminotransferase; γ-GTP, gamma-glutamyl transferase; AFP, alpha-fetoprotein; RVR, rapid virological response; EVR, early virological response; SVR, sustained virological response.
Comparison of SVR patients with non-SVR patients among previously treated patients
Next, we compared 18 SVR patients with 11 non-SVR patients among the previously treated patients (Table 2). The platelet count of SVR patients tended to be higher than that of non-SVR patients (P = 0.061). We did not see any differences in the baselines of other factors and treatment responses (Table 2). In the 18 SVR patients previously treated, 3 received conventional interferon monotherapy, 5 peginterferon alfa-2a monotherapy, 7 peginterferon alpha-2b plus ribavirin, 2 peginterferon alfa-2a plus ribavirin and 1 with details unknown. In the 11 non-SVR patients previously treated, 5 received peginterferon alfa-2a monotherapy, 5 peginterferon alpha-2b plus ribavirin, and 1 peginterferon alfa-2a plus ribavirin. Concerning previous treatment response of the 29 previously treated patients, 18 were relapsers, 7 non-responders, and 4 had details unknown. In the 18 SVR patients, 12 were relapsers, 4 non-responders, and 2 had details unknown. In 11 non-SVR patients, 6 were relapsers, 3 non-responders, and 2 had details unknown.
Baseline and demographic characteristics of SVR- and non-SVR-retreated patients
| SVR | Non-SVR | P-value* | |
|---|---|---|---|
| Number of patients | 18 | 11 | N.S. |
| Age (years) | 60.0 ± 10.0 | 60.3 ± 6.3 | N.S. |
| Gender (male/female) | 8/10 | 7/3 | N.S. |
| Body mass index (kg/m2) | 26.0 ± 3.4 | 26.5 ± 4.0 | N.S. |
| HCV RNA (log IU/mL) | 5.5 ± 1.9 | 5.5 ± 2.1 | N.S. |
| ALT (IU/L) | 57.8 ± 50.7 | 55.6 ± 52.8 | N.S. |
| γ-GTP (IU/L) | 46.0 ± 40.7 | 30.4 ± 17.6 | N.S. |
| AFP (ng/mL) | 5.7 ± 3.4 | 6.2 ± 5.7 | N.S. |
| Leukocyte count (/mm3) | 4940 ± 1670 | 4670 ± 940 | N.S. |
| Hemoglobin (g/dL) | 14.0 ± 1.6 | 13.6 ± 2.0 | N.S. |
| Platelet count (x104/mm3) | 16.2 ± 5.2 | 12.6 ± 4.1 | 0.061 |
| Treatment response | |||
| Duration of treatment (~24/48/72 wks) | 6/11/1 | 3/7/1 | N.S. |
| RVR rates, % | 44.4 (8/18) | 18.1 (2/11) | N.S. |
| HCV RNA negativity at 8 wks | 88.8 (16/18) | 66.6 (6/9) | N.S. |
| EVR rates, % | 88.8 (16/18) | 88.8 (8/9) | N.S. |
| Adherence (≥80/≥80/≥80), yes | 44.4 (8/18) | 54.5 (6/11) | N.S. |
Data are expressed as mean ± SD. *P-value, between groups with and without SVR by Student's t-test or chi-square test; N.S., not statistically significant; ALT, alanine aminotransferase; γ-GTP, gamma-glutamyl transferase; AFP, alpha-fetoprotein; RVR, rapid virological response; EVR, early virological response; SVR, sustained virological response; adherence was classified according to the previous report [19].
Previous treatment response and SVR rates in HCV genotype 2 retreated patients
The relationship between previous treatment response and SVR rates of HCV genotype 2 retreated patients is shown in Table 3. In 1 patient previously treated with peginterferon plus ribavirin and non-response, treatment was discontinued due to side effects by ~8 weeks and SVR was not obtained. Of 13 patients previously treated with peginterferon plus ribavirin who had relapsed, 2 discontinued treatment due to side effects by ~8 weeks.
Female cases retreated, in whose sera a single very low-titer fluctuation of HCV RNA from negative to positive was detected after HCV RNA had been undetected
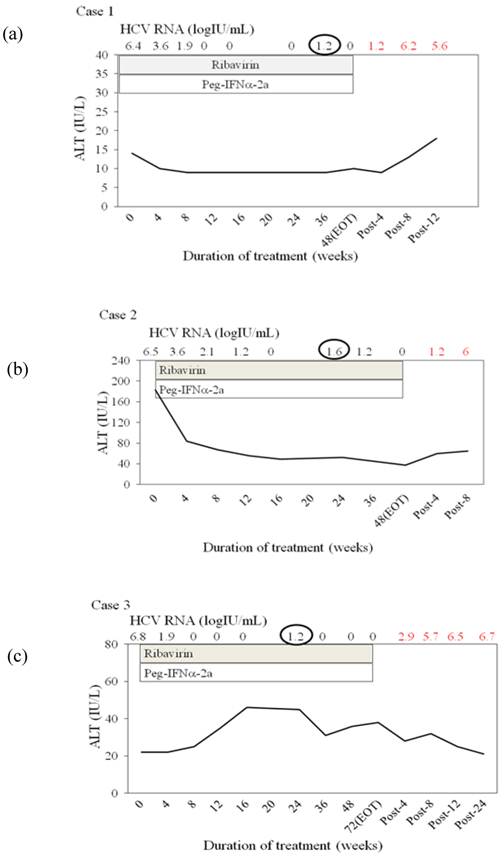
Furthermore, we tried to determine the clinical features of non-SVR HCV genotype 2 patients retreated with peginterferon alfa-2a plus ribavirin. We noticed 3 females retreated with peginterferon alfa-2a plus ribavirin and resulting in non-SVR, in whose sera a single very low-titer fluctuation of HCV RNA from negative to positive was detected after HCV RNA had been undetected (Figure 1). HCV RNA finally relapsed in all 3 cases. Treatment with SOC might need to be stopped in HCV genotype 2 female patients with these features.
Previous treatment response and SVR rates in 25 retreated patients
| Number of patients | Previous treatment (Treatment response) | Formula of retreatment | SVR rates (%) |
|---|---|---|---|
| 6 | Peginterferon alfa-2a (NR) | Peginterferon alfa-2a plus ribavirin (~24wks) | 66.6 |
| 1 | Peginterferon plus ribavirin (NR) | Peginterferon alfa-2a plus ribavirin (~24wks) | 0 |
| 5 | (Peg-)interferon (relapse) | Peginterferon alfa-2a plus ribavirin (~48wks) | 60 |
| 13 | Peginterferon plus ribavirin (relapse) | Peginterferon alfa-2a plus ribavirin (24~48wks) | 69.9 |
NR, non-response
DISCUSSION
In the present study, we focused on the virological response in HCV genotype 2-infected Japanese patients retreated with peginterferon alfa-2a plus ribavirin. We did not observe any differences in baseline background between SVR patients retreated and non-SVR patients retreated, although we must admit that the number of patients was small. However, during this study, we did find 3 females who did not obtain SVR by the retreatment and had unique features. That is, in their sera, a single very low-titer fluctuation of HCV RNA from negative to positive was detected after HCV RNA had been undetected (Figure 1). These 3 cases did not discontinue peginterferon alfa-2a or ribavirin. In Figure 1, cases 1 and 2 had reduced peginterferon alfa-2a but not reduced ribavirin. On the other hand, case 3 had reduced ribavirin due to anemia, but did not have a reduction of peginterferon alfa-2a. In cases 2 and 3, adherence (≥80/≥80/≥80) [19] based on the calculation at 48 weeks was not lower. These 3 cases were relapsers and seemed different from non-responders having anti-interferon-alfa neutralizing antibody [20]. We do not know the exact reasons at this time.
Three females retreated with peginterferon alfa plus ribavirin and resulting in non-sustained virological response, in whose sera a single very low-titer fluctuation of HCV RNA from negative to positive was detected after HCV RNA had been undetected. (a) Case 1, 68 years, female, IL28Brs8099917 TT. She was previously treated with peginterferon alfa-2a for 48 weeks, with details unknown. (b) Case 2, 58 years, female, IL28Brs8099977, not determined. She was previously treated with peginterferon alfa-2a for 48 weeks, with relapse. (c) Case 3, 58 years, female, IL28Brs8099917 TG. She was previously treated with peginterferon alfa-2b plus ribavirin, with details unknown. HCV RNA was determined by COBAS TaqMan HCV test (Roche), with levels ranging from 1.2 to 7.8 log IU/mL [16].
In the present study, 44% of patients had rapid virological response (RVR) and 89% of the patients had EVR (cEVR) in the retreated genotype 2 chronic hepatitis C patients with an SVR (Table 2). These results were concordant with previous studies. However, 89% of the non-SVR patients also had EVR (Table 2). Among the 8 non-SVR patients, 3 had lower adherence (≥80/≥80/≥80) (data not shown). In the present study, the adherence rates were quite low (44% in patients with SVR, and 54% in patients without SVR). In certain cases, lower adherence may be one of the reasons for non-SVR.
For HCV genotype 1 patients, direct acting antivirals (DAAs) such as telaprevir and boceprevir have been available in clinical practice [7, 21-23]. The addition of telaprevir or boceprevir to peginterferon plus ribavirin resulted in significantly higher rates of SVR in previously treated patients with chronic HCV genotype 1 infection [7, 21-23]. It will require more time until the more frequent use of DAAs for the treatment of HCV genotype 2 patients will become possible [24, 25]. Until then, we have to retreat HCV genotype 2-infected patients with peginterferon alfa-2a plus ribavirin for 24-48 weeks.
Recently, it was reported by several groups that genetic variations in IL28B-SNP predict HCV genotype 1 treatment-induced viral clearance [7, 26-29]. Yu et al. [30] reported that rs8099917 TT genotype is significantly independently predictive of RVR, which is the single best predictor of SVR, in Asian HCV genotype 2 patients. Further study will be needed.
In conclusion, we showed that retreatment of HCV genotype 2-infected Japanese patients with peginterferon alfa-2a plus ribavirin for 24-48 weeks resulted in 60 to 66.6% SVR in patients previously treated with (peg-)interferon monotherapy and in 69.9% SVR in relapsers previously treated with peginterferon plus ribavirin, which supports the previous reports [12, 13]. Attention should be paid to certain patients with unique features. Selection of patients according to previous treatment could lead to optimal therapy in HCV genotype 2 treatment-experienced patients.
Acknowledgements
We thank Dr Yutaka Yonemitsu, Dr Fumihiko Kanai, Dr Akinobu Tawada, Dr Nobuyuki Sugiura, Dr Rintaro Mikata, Dr Tetsuhiro Chiba, Dr Motohisa Tada, Dr Motohide Takashi, Dr Kenichi Fukai, Dr Yasushi Maru, Dr Takeshi Nihei, Dr Norio Kikuchi, Dr Noritomo Shimada, Dr Yasuo Hirai, Dr Shuuichi Saito, Dr Shinichi Hino, Dr Shousuke Iwama, Dr Masaaki Saito, Dr Hiroshige Kojima, Dr Michio Kimura, Dr Kazuhiko Kita, Dr Susumu Nakahori, Dr Shinichi Sato, Dr Yutaka Natsuki, Dr Hidetaka Terabayashi, Dr Masahiko Sanada, Dr Noriaki Suzuki, Dr Ryosaku Azemoto, Dr Hideki Takanashi, Dr Katsumi Doai, Dr Shinnen Kin, Dr Akito Nozaki, Dr Satoru Kaneda, Dr Michikazu Abe, Dr Hikaru Nagahara, Dr Yoko Hoshino, Dr Kinki Rin and all other investigators for coordinating with this work. This study was supported by research grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (TK, SN and TT), a grant from the Ministry of Health, Labour and Welfare of Japan (OY), and a Special Coordination Fund for Promoting Science and Technology of the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government (TK).
CONFLICT OF INTEREST
Dr. Tatsuo Kanda reports receiving lecture fees from Chugai Pharmaceutical, MSD, and Ajinomoto, and Prof. Osamu Yokosuka receiving grant support from Chugai Pharmaceutical, Bayer, MSD, Daiichi-Sankyo, and Mitsubishi Tanabe Pharma.
ABBREVIATIONS
cEVR: Complete early virological response; DAA: Direct-acting antiviral; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; IL28B: Interleukin-28B; RVR: Rapid virological response; SNP: Single nucleotide polymorphism; SD: Standard deviation; SOC: Standard of care; SVR: Sustained virological response.
References
1. Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26(3 Suppl 1):34S-38S
2. Kanda T, Imazeki F, Mikami S. et al. Occurrence of hepatocellular carcinoma was not a rare event during and immediately after antiviral treatment in Japanese HCV-positive patients. Oncology. 2011;80:366-372
3. Simmonds P, Bukh J, Combet C. et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962-973
4. Takano S, Yokosuka O, Imazeki F. et al. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650-655
5. Mangia A, Santoro R, Minerva N. et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609-2617
6. Shiffman ML, Suter F, Bacon BR. et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;357:124-134
7. Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548-561
8. Yu ML, Dai CY, Huang JF. et al. A randomized study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis C. Gut. 2007;56:553-559
9. Lagging M, Langeland N, Pedersen C. et al. Randomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology. 2008;47:1837-1845
10. Yokosuka O, Iwama S, Suzuki N. et al. High sustained virologic response rate after interferon monotherapy in Japanese hepatitis C patients with a low HCV RNA titer and/or HCV genotype 2. A prospective study. Intervirology. 2004;47:328-334
11. Omata M, Kanda T, Yu ML. et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6:409-435
12. Sherman M, Yoshida EM, Deschenes M. et al. Peginterferon alfa-2a (40KD) plus ribavirin in chronic hepatitis C patients who failed previous interferon therapy. Gut. 2006;55:1631-1638
13. Poynard T, Colombo M, Bruix J. et al. Peginterferon alfa-2a and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136:1618-1628
14. Kanda T, Imazeki F, Azemoto R. et al. Response to peginterferon-alfa 2b and ribavirin in Japanese patients with chronic hepatitis C genotype 2. Dig Dis Sci. 2011;56:3335-3342
15. Etoh R, Imazeki F, Kurihara T. et al. Pegylated interferon-alfa-2a monotherapy in patients infected with HCV genotype 2 and importance of rapid virological response. BMC Res Notes. 2011;4:316
16. Kanda T, Imazeki F, Yonemitsu Y. et al. Quantification of hepatitis C virus in patients treated with peginterferon-alfa 2a plus ribavirin treatment by COBAS TaqMan HCV test. J Viral Hepat. 2011;18:e292-297
17. Tsukiyama-Kohara K, Yamaguchi K, Maki N. et al. Antigenicities of Group I and II hepatitis C virus polypeptides—molcular basis of diagnosis. Virology. 1993;192:430-437
18. Tanaka T, Tsukiyama-Kohara K, Yamaguchi K. et al. Significance of specific antibody assay for genotyping of hepatitis C virus. Hepatology. 1994;19:1347-1353
19. McHutchison JG, Manns M, Patel K. et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061-1069
20. Matsuda F, Torii Y, Enomoto H. et al. Anti-interferon-α neutralizing antibody is associated with nonresponse to pegylated interferon-α plus ribavirin in chronic hepatitis C. J Viral Hepat. 2012;19:694-703
21. McHutchison JG, Manns MP, Muir AJ. et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292-1303
22. Zeuzem S, Andreone P, Pol S. et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428
23. Bacon BR, Gordon SC, Lawitz E. et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217
24. Foster GR, Hezode C, Bronowicki JP. et al. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology. 2011;141:881-889
25. Mangia A, Mottola L. What's new in HCV genotype 2 treatment. Liver Int. 2012;32(Suppl 1):135-140
26. Ge D, Fellay J, Thompson AJ. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401
27. Tanaka Y, Nishida N, Sugiyama M. et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109
28. Suppiah V, Moldovan M, Ahlenstiel G. et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104
29. Miyamura T, Kanda T, Nakamoto S. et al. Hepatic STAT1-nuclear translocation and interleukin 28B polymorphisms predict treatment outcomes in hepatitis C virus genotype 1-infected patients. PLoS One. 2011;6:e28617
30. Yu ML, Huang CF, Huang JF. et al. Role of interleukin-28B polymorphisms in the treatment of hepatitis C virus genotype 2 infection in Asian patients. Hepatology. 2011;53:7-13
Author contact
![]() Corresponding author: Tatsuo Kanda, MD, PhD, Associate Professor, Department of Gastroenterology and Nephrology, Graduate School of Medicine, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba 260-8677, Japan. Tel.: +81-43-226-2086, Fax: +81-43-226-2088; E-mail: kandat-cibac.jp
Corresponding author: Tatsuo Kanda, MD, PhD, Associate Professor, Department of Gastroenterology and Nephrology, Graduate School of Medicine, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba 260-8677, Japan. Tel.: +81-43-226-2086, Fax: +81-43-226-2088; E-mail: kandat-cibac.jp

 Global reach, higher impact
Global reach, higher impact